The update to the Community-led Duchenne Guidance for FDA is well underway and on track to be submitted to the agency in the beginning of 2022.
As you know, in 2014, we came together as a community, creating and submitting to the FDA the first-ever patient advocacy-initiated Duchenne Guidance for developing therapies to help accelerate drug development. In 2018, the FDA finalized their own Duchenne Guidance, and the drug development pipeline subsequently expanded. Since 2014 a lot has changed. Our community has experienced trial and regulatory successes with five drug approvals, as well as difficult disappointments with several study terminations. As the field has evolved, so has our understanding and learnings about what to do differently moving forward. That is why we are updating the Guidance so that trials are run as efficiently as possible and we take the learnings from past and apply them moving forward.
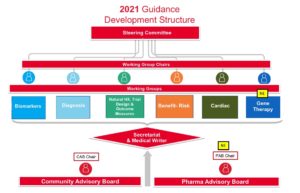
The process of updating the Guidance has involved over 100 stakeholders including patients, caregivers, clinicians, researchers, drug companies, genetic counselors, and regulatory experts. This unprecedented effort brings together the leading experts in Duchenne serving on working groups in the following areas:
- Benefit Risk Preferences and Patient Experience
- Biomarkers
- Diagnosis
- Cardiac
- Gene Therapy
- Natural History, Trial Design, Outcome Measures
Members of each working group can be viewed here.
Two advisory boards are also involved in the update overseeing the process and commenting on each section of the Guidance. A Pharmacy Advisory Board comprised of 21 companies currently developing therapies for Duchenne and Becker, and a Community Advisory Board that includes 20 Duchenne partner organizations, The Everylife Foundation for Rare Diseases, and the Muscular Dystrophy Association, as well as 18 Individuals members of our community.
| Community Advisory Board | Pharmacy Advisory Board |
|---|---|
| ActionDuchenne | Antisense Therapeutics Limited |
| Best Day Ever Foundation | Astellas GT |
| Charley's Fund | Avidity Bio |
| Coalition Duchenne | Cumberland Pharma |
| Cure Duchenne | Daiichi Sankyo |
| Duchenne Australia | Dyne |
| Duchenne UK | Edgewise |
| EveryLife Foundation for Rare Diseases | Eli Lilly |
| JB's Keys to DMD | EntradaTX |
| Jesse's Journey | Empirium |
| Jett Foundation | FibroGen |
| Kindness Over Muscular Dystrophy | Pfizer |
| Little Hercules Foundation | PTC Bio |
| MD Canada | Regenex Bio |
| Muscular Dystrophy Association | Reveragen |
| My DMD Hero | Roche |
| ParentProject Onlus | Santhera |
| Powers Promise | Sarepta |
| Save Our Sons | Solid Bio |
| Team Joseph | TRINDS |
| The Hope for Gus Foundation | Vertex |
| Walking Strong |
We will keep the community informed as we continue to make progress. If you are a community member interested in participating on the CAB, there is still time to join. Please contact PPMD’s Ryan Fischer at ryan@parenprojectmd.org for more information, including required time commitments.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

