Moving Forward Together was the theme of PPMD’s 2020 Duchenne Healthcare Professionals’ Summit held late last month. It was an appropriate theme considering the audience of healthcare professionals in attendance who have consistently demonstrated their willingness to work collaboratively to move the field of Duchenne care. To date, there are 27 PPMD Certified Duchenne Care Centers (CDCCs) and in this year’s meeting, all 27 CDCCs were represented with 12 additional institutions participating, expressing interest in joining this network. These centers provide optimal standardized care and services to Duchenne patients, but these experts rarely have the opportunity to spend time together, to collaborate across centers to share information, and learn from one another. This is the purpose of the Summit – to encourage collaborations across centers, to think about gaps in knowledge, to share case studies, to learn from each other with the goal of continually improving care for every person and every family with Duchenne.
Newborn Screening
For those already diagnosed with Duchenne, newborn screening may not feel very relevant to today. But we have to think of every future family receiving the diagnosis of Duchenne and hope for a day when a newly diagnosed family hears ‘and here’s how we treat Duchenne, here is the therapy that will preserve their muscles and their heart.’
For this reason, PPMD launched a two-year pilot program to screen newborns in New York state for Duchenne. The first baby was screened in October 2019. The process is to conduct this pilot program, apply to the RUSP (Recommended Uniform Screening Panel), and include Duchenne on the list of disorders that the Secretary of Health and Human Services recommends to states to screen as part of their state universal screening program.
Early diagnosis. Early Treatment. Hope.
Natural History: Measuring and Modeling
We sometimes think of the natural history – a look at the progression of Duchenne and following the ability to predict the trajectory – as static. But natural history is ongoing, impacted by many factors to include care, interventions, therapies, and environment to name a few. Learnings from evolving natural history studies are critically important to understand the impact of a specific therapy, to understand safety and efficacy, and to improve our ability to ensure access to therapies.
Various outcome measures are likely familiar. The North Star Ambulatory Assessment (NSAA) and Performance of the Upper Limb (PUL) are commonly measured, but HOW they are measured can vary from institution to institution, from person to person. Working on better ways to collect these measures consistently is critical. We also need to explore, consider, and develop more sensitive measures. The sequence in which these measures are collected will improve natural history studies, clinical assessments, and clinical trials.
Quality of Life: For Patients and for Providers
We often talk about Quality of Life (QoL) and ways to measure it, because it is important to understand the factors that contribute to preserving and improving the quality of our lives. This is one of the areas we think about at PPMD. We are working hard to learn more about how Duchenne affects the brain, and how we can better manage problems with learning, behavior, anxiety, and depression. The attendees heard about a wide range of psychosocial symptoms seen in individuals with Duchenne, and discussed new ways to better assess, evaluate, and treat these problems.
Compassion fatigue may feel like an odd term, but we all feel the weight of Duchenne. Healthcare providers are no different. Patricia Smith presented on compassion fatigue, and how this can affect everyone who cares for families with Duchenne. The bottom line for all of us is to ‘put on the oxygen mask first, before taking care of others.’ We all need to be mindful of our own mental health if we are to be the best we can be – healthcare professional, parent, family member, or person living with Duchenne.
Duchenne Case Studies
Case studies are for the purpose of exploring problems that clinicians see in clinic or in the emergency department that are unusual, complicated, or have not been seen before. Discussing this within the group raises awareness and enables group discussion about how to best manage these cases. It is shared learning and a good way to evaluate whether these circumstances are becoming common and to seek solutions.
The Current State of Duchenne Clinical Trials
Clinical Trials = Hope. We have a robust clinical trials pipeline and many of the CDCCs are sites for these trials, but no one center is a site for every single study. It was important to update all attendees, leveling the playing field so that everyone in attendance was well-informed, well-aware, and able to discuss with families what trials exist, which trials could be considered, the requirements, and potential burden. Gene therapy studies are underway and there is a great deal of enthusiasm and discussion around protocols, screening, immunity, and next steps. Breakout groups and panels focused on looking ahead in gene therapy, discussing both immunity and re-dosing issues.
Dr. Janet Woodcock, the director of the Center for Drug Evaluation and Research (CDER) at the FDA, presented her views on improving clinical trial design, discussing the potential to build a platform trial using a master protocol. Dr. Woodcock touched on benefits of a master protocol as an innovative option to drive therapy development. The goal of a platform trial would be to accelerate studies, expose fewer patients to placebo, and get to a ‘go or no-go’ more quickly. This means saving TIME in a disease that waits for no one.
One critical discussion involved the balancing act – how to balance opinions about specific trials with the need to recruit for all – while also balancing institutional commitments to industry needs and always keeping the family’s wishes as THE priority.
The Duchenne Registry and Interchange (DORI)
The Interchange – a 360 look. PPMD’s Ryan Fischer led a panel to discuss the new Duchenne Registry App, the Duchenne Outcomes Research Interchange, and the potential to improve research and our understanding of Duchenne by using patient data. Ryan was joined by Dr. Mary Schroth from Cure SMA, who shared early success in electronic health record (EHR) extraction used to build a more comprehensive data set to improve outcomes. Both PPMD and Cure SMA are utilizing the same partner, Prometheus Research, in order to un-silo data from hospitals and put it to use to inform our understanding of disease progression. If you haven’t done so already, be sure to register on the new PPMD Duchenne Registry App. The more participants we have, the better the data.
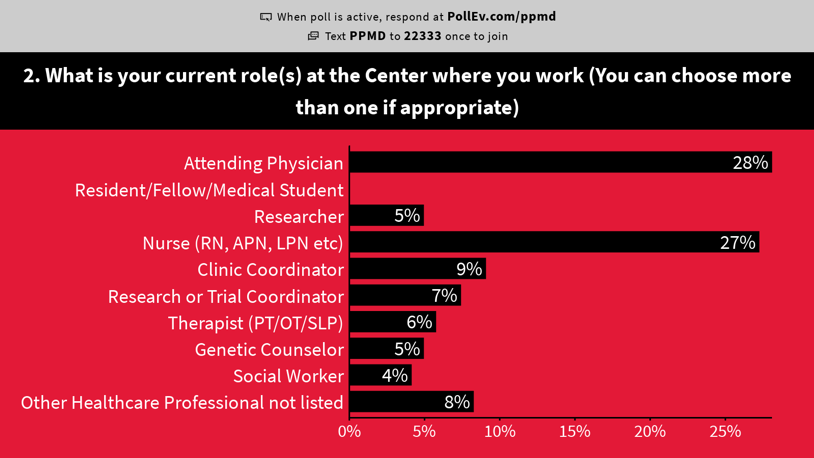
Live Polling
Over the course of the three day meeting, PPMD did live polling on topics related important to healthcare professionals. This data helps us have a better understanding of how different providers (nurses, coordinators, doctors, PTs, etc.) think and feel about a range of issues around care and drug development. Click here to view our polling results.
Moving Forward Together
We are moving forward…together! PPMD is grateful to have such a compassionate, determined, expert group of healthcare providers, researchers, and industry representatives in our Duchenne community. It was clear that everyone in attendance was motivated to learn, collaborate with others, and continue to evolve their practice to reach our ultimate goal: to continue moving forward together.
We look forward to another successful meeting in January 2021 and to see a lot of these same folks at PPMD’s Annual Conference in June!




 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy