Solid Biosciences has shared an update with the community this morning, including a dosing update in the IGNITE DMD Phase I/II clinical trial, promising long-term biopsy data from prior patients dosed with SGT-001 in the high dose cohort at 2E14 vg/kg, and a pipeline update on their new preclinical product, SGT-003.
It was reported that two patients have been dosed at 2E14 vg/kg since Solid came off clinical hold; one patient did have a drug-related serious adverse event (SAE), though at 30 days their labs were trending towards or returned to normal. Solid also shared biopsy data collected from 1-2 years in patients previously treated in the high dose cohort of 2E14 vg/kg, the expression of micro-dystrophin in these patients indicate durability of the construct. Additionally, recruitment of dystrophin associated proteins support the functionality of the micro-dystrophin protein being generated.
Finally, Solid has also announced the advancement of a next-generation gene therapy program for Duchenne, SGT-003, a preclinical candidate that combines a novel and rationally designed capsid candidate, that may help to increase efficacy, with Solid’s proprietary nNOS-containing micro-dystrophin.
Read Solid’s Community Letter:
Dear Duchenne Community,
This morning we issued a press release providing a business update, including a dosing update in the IGNITE DMD Phase I/II clinical trial and promising long-term biopsy data from prior patients dosed with SGT-001 in the high dose cohort at 2E14 vg/kg. Additionally, we announced the advancement of a next-generation gene therapy program for Duchenne.
IGNITE DMD Clinical Trial Update
We have continued patient dosing in IGNITE DMD with SGT-001 using Solid’s improved manufacturing process and under an amended clinical protocol designed to enhance patient safety. As we previously shared, Patient 7 was dosed safely and continues to do well. We dosed our eighth patient who experienced an inflammatory response that was classified as a serious adverse event (SAE) and considered by the investigator to be drug related. It is important to note this SAE was described in our investigators brochure and not considered unexpected. We are pleased to share this patient is home with his family and continues to improve with laboratory values back to normal or continuing to trend towards normal at his 30-day post dosing visit.
As part of our clinical mitigation strategy, the status of both patients dosed under the amended protocol have been shared with our Data Safety Monitoring Board (DSMB) as well as the Food and Drug Administration (FDA). We are carefully examining the full details of the clinical course of these patients and continuing our discussions with these two bodies. Importantly, no new drug-related safety findings have been identified in Patients 1 through 6 who have post dosing periods of 1.5 years to more than 3 years.
IGNITE DMD Long Term Biopsy Data Update
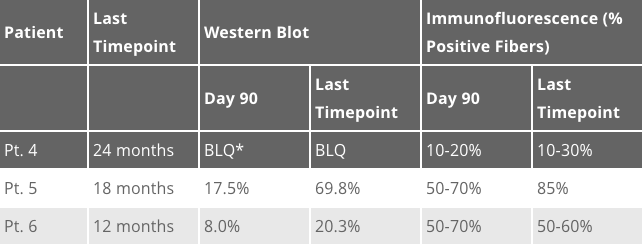
We are excited to share long-term biopsy data collected from patients 4-6 at the 2E14 vg/kg dose level on today’s conference call and at the American Society for Gene & Cell Therapy (ASGCT) annual meeting this afternoon. Analyses of the needle biopsies, taken 2 years, 1.5 years and 1-year post-dosing, respectively, indicate evidence of durable and widespread expression of the microdystrophin protein. The long-term results are consistent with the Day 90 data previously reported and continue to demonstrate the functionality of the SGT-001 microdystrophin, as highlighted by the recruitment of key dystrophin associated proteins: beta-sarcoglycan and neuronal Nitric Oxide Synthase (nNOS). The long-term muscle biopsy results were analyzed by two methods, Western Blot and Immunofluorescence (IF), and are provided in the table below:
*Below the limit of quantification
In summary, the Western Blot data indicate that expression was maintained in Patient 4 and increased compared to the Day 90 biopsies in Patient 5 and 6. IF data show microdystrophin function via continued localization to the muscle membrane, with the percent positive fibers remaining comparable to the Day 90 biopsies in all three patients.
Further analyses of the muscle biopsies indicate that sustained microdystrophin protein expression and function resulted in membrane stabilization, evidenced by minimal progression of muscle deterioration since the Day 90 timepoint. These long-term improvements are potentially supportive of the recently reported positive trends in the clinical biomarker and functional data from the IGNITE DMD trial.
Pipeline Update
Today we announced the advancement of a next-generation DMD microdystrophin gene transfer program, SGT-003. This program is an internally developed preclinical candidate leveraging Solid’s expertise in gene therapy and muscle biology. Data presented at the ASGCT meeting demonstrate that Solid has successfully developed a library of novel capsids that show increased muscle tropism, decreased liver biodistribution, that drive improved efficiency compared with AAV9. SGT-003 is a preclinical candidate that combines a novel and rationally designed capsid candidate with Solid’s proprietary nNOS-containing microdystrophin.
Conference Call Information
We will host a conference call at 8:30 am EST today to discuss the program update. We invite you to listen by dialing +1 866-763-0341 (domestic) or +1 703-871-3818 (international) five minutes prior to the start of the call and providing the passcode 4772539. A listen-only webcast of the conference call can also be accessed through the “Investors” tab on the Solid Biosciences website, www.solidbio.com, and a replay of the call will be available for approximately six weeks after the call.We are extremely encouraged by the long-term biopsy data collected to date and the benefit we hope this will bring to patients that have been dosed with SGT-001. We are looking forward to providing an update on the status of screening and enrollment in the IGNITE DMD clinical trial.
#TogetherWeAreSolid
Sincerely,
Your Solid Biosciences Team



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy