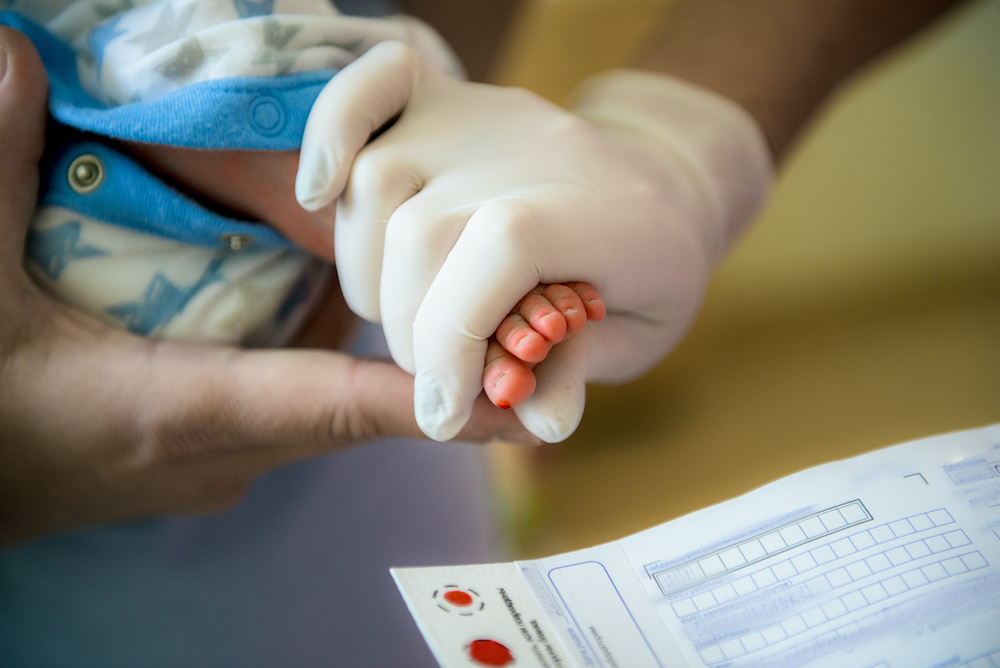
PPMD’s Newborn Screening Pilot was recently highlighted at the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) meeting.
The ACHDNC advises the US Department of Health and Human Services Secretary about which conditions should be considered for newborn screening. The Committee has two scheduled meetings this year, and during their meeting earlier this month, testimony was given on PPMD’s ongoing pilot for Duchenne newborn screening in New York state.
The pilot program was designed to set up, validate, and conduct a consented pilot screen for infants born at select hospitals in New York State. The pilot utilizes tools, resources, and expertise at PPMD and the Newborn Screening Translational Research Network (NBSTRN), a National Institute of Child Health and Human Development, National Institutes of Health (NICHD/NIH) program housed at the American College of Medical Genetics and Genomics (ACMG), and the New York State Department of Health.
The pilot is funded by PTC Therapeutics, Sarepta Therapeutics, PerkinElmer, Pfizer, Inc., Solid Biosciences, and Wave Life Sciences. The pilot is led by a consortium of Duchenne industry partners with a commitment to early diagnosis and intervention in Duchenne and guided by a Steering Committee comprised of representatives from federal agencies, provider groups, and representatives from key Duchenne stakeholder communities.
The testimony was very well received, and the Committee chair had specific questions about the Duchenne therapies. The opportunity to share information about our pilot with the Committee keeps Duchenne at the forefront. These connections and conversations with the Committee will hopefully enable a successful application to add Duchenne to the Recommended Uniform Screening Panel (the RUSP) for newborn screening in the future.
Read Testimony


 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

