Good news out of Japan! NS Pharma, Inc., announced that their parent company, Nippon Shinyaku Co., Ltd., has received approval for viltolarsen in Japan for people with Duchenne amenable to exon 53 skipping therapy.
Viltolarsen was granted Priority Review in the US with a decision expected in the third quarter of 2020. We look forward to continued updates from NS Pharma regarding the progress of this potential therapeutic option for those amendable to exon 53 skipping.
Read NS Pharma’s Announcement
Marketing authorization in Japan of VILTEPSO® Intravenous Infusion 250 mg for the treatment of Duchenne muscular dystrophy
KYOTO, Japan Mar. 25, 2020 – Nippon Shinyaku Co., Ltd. (President, Shigenobu Maekawa) announced today that the Ministry of Health, Labour and Welfare (MHLW) has approved VILTEPSO® Intravenous Infusion 250 mg (viltolarsen, previously NS-065/NCNP01) for the treatment of patients with Duchenne Muscular Dystrophy (DMD) who are amenable to exon 53 skipping therapy. This represents the first regulatory approval for viltolarsen in the world and the first approved DMD treatment other than steroids in Japan.
DMD is a progressive form of muscular dystrophy that occurs primarily in males. DMD causes progressive weakness and loss (atrophy) of skeletal, cardiac, and pulmonary muscles due to a deficiency of normal dystrophin, a protein involved in constructing the framework of muscle cells.
VILTEPSO® Intravenous Infusion 250 mg is a morpholino antisense oligonucleotide, which was co-discovered by Nippon Shinyaku and National Center of Neurology and Psychiatry (NCNP: Kodaira City, Tokyo; President, Hidehiro Mizusawa, Executive Director, Shin’ichi Takeda). It is designed to increase dystrophin production by binding to exon 53, an exon next to the dystrophin gene which results in the translation of the mRNA into a shortened dystrophin protein that contains essential functional portions. In Japan, VILTEPSO® Intravenous Infusion 250 mg was granted SAKIGAKE designation of MHLW in October, 2015, Orphan drug designation in August in 2019, and designation of Conditional Early Approval System in October in 2019. In the U.S, viltolarsen was granted a Rare Pediatric Disease Designation, Orphan Drug Designation, and a Fast Track Designation.
Viltolarsen has not yet been approved in the U.S.
Nippon Shinyaku continues to focus their efforts on developing treatments for intractable and rare diseases, including DMD.
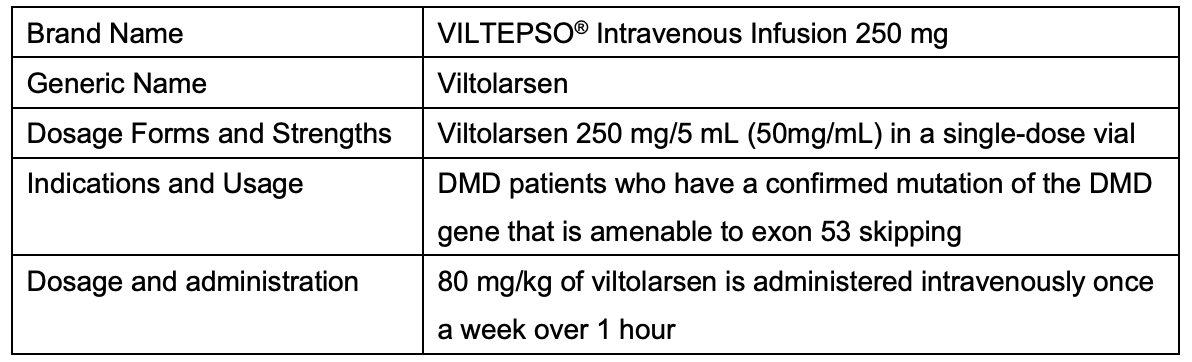
Summary of VILTEPSO
Brand Name VILTEPSO® Intravenous Infusion 250 mg Generic Name Viltolarsen Dosage Forms and Strengths Viltolarsen 250 mg/5 mL (50mg/mL) in a single-dose vial Indications and Usage DMD patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping Dosage and administration 80 mg/kg of viltolarsen is administered intravenously once a week over 1 hour
Nippon Shinyaku conducted a phase 1/2 study and submitted NDA in September 2019. The North America P2 clinical study was conducted by NS Pharma, Inc. (President, Tsugio Tanaka), a wholly owned subsidiary of Nippon Shinyaku. Viltolarsen was granted Rare Pediatric Disease Designation, Orphan Drug Designation, and Fast Track designation in the U.S. Its New Drug Application (NDA) was granted Priority Review by the FDA with an anticipated action date in the third quarter of 2020. While viltolarsen remains under review by the FDA, NS Pharma continues to study the efficacy and safety of viltolarsen in the Phase 3 RACER53 trial initiated. To meet our commitment to the FDA and the DMD community, we initiated our confirmatory Phase 3 trial shortly after the completion of our NDA submission to the FDA.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy