
On Saturday, April 28, PPMD had the pleasure of hosting the End Duchenne Tour in St. Paul, MN at Gillette Children’s Specialty Healthcare. This was our first tour visit to St. Paul and we are thrilled to have hosted over 60 people with the great team at Gillette.
Join the Fight
Families attending the End Duchenne Tour stop in St. Paul learned more about PPMD’s Race to End Duchenne program, including the Medtronic Twin Cities Marathon Weekend on October 6 and 7. Click here to find out more about this and other Race to End Duchenne events.
Jack Kirley, a local father, spoke about PPMD’s Connect Minnesota group (previously FACES). If you live in Minnesota, this group is a great way to connect with local families for support, friendship and fun. Learn more about Connect Minnesota and other Connect groups around the country.
The Decode Duchenne program, offering free genetic testing and counseling for people with Duchenne, is now available for carriers – learn more!

Care Highlights
- Pat Furlong discussed the new standard of care guidelines for Duchenne that were published earlier this year, as well as our network of Certified Duchenne Care Centers, of which Gillette is one. Pat also discussed the updated care sections on the new PPMD website.
- Keith Cavanaugh, MD of Gillette focused on the new pulmonary guidelines for Duchenne. He discussed the importance of pulmonary evaluations starting at age 6 and then annually. He also reminded parents not to forget the “basics” – immunizations, frequent hand washing, and no smoking. (Download presentation slides)
- Kathy Lindstrom APRN, CNP from Gillette discussed transition care through adulthood. She reminded everyone that transition is complex and occurs over years. She also reviewed the 6 domains of planning for transition – healthcare, activities of daily living, education/employment, housing, transportation, and relationships. Check out gottransition.org for more information. (Download presentation slides)
- The final care talk was presented by three practitioners from the Adult Clinic at Gillette – a PM&R physician, an occupational therapist who also discussed physical therapy, and a speech therapist. They covered the following topics: Independence, Mobility, Communications, and Function. (Download presentation slides)

Industry Updates
- From Summit, Michelle Avery, PhD spoke on ezutromid, an oral utrophin modulator. Their Phase 2 trial, PhaseOut DMD, is currently ongoing with 40 patients enrolled. After 24 weeks, they have seen stabilization of muscle membranes, and significant decrease in muscle damage and muscle inflammation. They are waiting on the 48 week results (Q3 2018) and they are starting to plan their next trial. If 48 week results are positive, they will likely apply for accelerated approval. (Download presentation slides)
- Brett Billmeyer, who works in Patent Advocacy at Sarepta, reviewed their robust pipeline. He emphasized their “next generation exon skipping” products which are on a PPMO backbone. The PPMO exon 51 skipping trial is actively recruiting participants. Brett also discussed their new Route 79 Duchenne Scholarship program. Ten scholarships of up to 10k will be awarded for the fall 2018 semester. Application deadline is May 31!
- Santhera was represented by Rebecca Persinger and she discussed the ongoing idebenone trial in Duchenne. Idebenone is an oral pill that works within the cells’ mitochondria as a potent antioxidant. The current SIDEROS trial is actively recruiting 266 males with Duchenne who are taking steroids. Gillette is a site. Rebecca also discussed their new website on respiratory health: TakeaBreathDMD.com (Download presentation slides)
- Marie Brace from PTC Therapeutics reviewed PTC’s 20 year history and their focus on oral therapies as well as their ongoing commitment to Duchenne. Marie reviewed both ataluren (which is currently in trial in the US) and Emflaza (which is now approved). She highlighted the PTC Cares program for patient support and assistance. She also discussed some of PTC’s other programs such as the Sibling Program.
- Rodger Kobes, MD, PhD from Pfizer discussed their two Duchenne therapies that are currently in trial. 1) Pfizer’s myostatin inhibitor (called domagrozumab) is in Phase 2 and results are expected possibly by September. 2) Pfizer’s mini-dystrophin gene therapy is in a Phase 1b trial. They are recruiting boys ages 5-12 at 4 sites in US (only 3 patients to start, then 6, then 12 total). The first patient was infused in March at Duke. This is incredibly exciting because a one-time infusion could completely change Duchenne, but this is a new technology with many unknowns. It is also likely that ~30% of boys will be ineligible for this treatment due to pre-existing antibodies to the viral capsid carrying the mini-dystrophin gene.
- Catabasis was represented by Dr. Joanne Donovan, who reviewed their therapy called edasalonexent, which is a steroid replacement without the steroid side effects. The MOVE DMD trial is an ongoing Phase 2 trial and there is an upcoming Phase 3 trial. So far edasalonexent has been “well tolerated without safety signals”, and in general, BMI, CK, and heart rate have decreased in participants. The Phase 3 trial will be for 4-7 year olds who are not on steroids or other investigational drugs. This will be a global study with multiple sites in the US, including at Gillette. Recruitment will begin later this year. (Download presentation slides)
- Brian Fedor from Capricor spoke about their CAP-1002 allogenic cardiosphere derived cells. These were originally developed for post-heart attack patients, but they have since been found to improve cardiac function and increase exercise capacity in mdx mice. Results of the HOPE-Duchenne trial were reviewed which included a reduction in cardiac scarring and increased regional systolic wall thickening. Results also indicate a functional benefit, and it was safe and well-tolerated. The HOPE-2 trial is now actively recruiting males 10 years or older at multiple sites across the US. Participants will receive 4 doses of CAP-1002 or placebo. (Download presentation slides)

PPMD Speakers
- In addition to Pat Furlong speaking on care, we had Ann Martin, a genetic counselor with PPMD, reviewing the genetics of Duchenne and the importance of knowing your mutation. Ann also reviewed the Decode Duchenne free genetic testing program, which is now available to carriers! And she showed highlights of The Duchenne Registry’s new website and features. Go to duchenneregistry.org to learn more.
- As mentioned above, Jack Kirley, a local father, spoke about PPMD’s Connect Minnesota group (previously FACES). Learn more here.
- Brian Denger, an active parent in our community, reviewed many of PPMD’s advocacy initiatives and discussed easy ways you can get involved either locally or on the national level. (Download presentation slides)

Race to End Duchenne
- Brian Denger also discussed PPMD’s Race to End Duchenne program (previously Run for Our Sons). Brian is an avid runner and he has participated in many PPMD runs in the past. This is a great way to meet others, get in shape, and raise money for Duchenne. Brian is available to help any parent or family member who is interested in giving this a try!
- For those in the St. Paul area, you can join the Race to End Duchenne team at the upcoming Medtronic Twin Cities Marathon Weekend on October 6 and 7. There are several race distances available and this is a great way to raise funds and awareness while doing something healthy for yourself. Learn more here.

Thank You!
Thank you to all the families who spent the day with us in St. Paul, and a special thanks to the Gillette staff who helped make the day possible. We would also like to thank Team Joseph and the Little Hercules Foundation who partnered with us for this event.
Our next End Duchenne Tour will be in Birmingham, AL on Saturday, June 2, 2018. We hope to see you there!
About the End Duchenne Tour
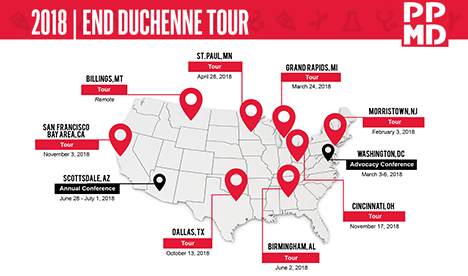
In an effort to reach every single family facing a Duchenne diagnosis in the U.S., PPMD has launched a multi-year community experience called the End Duchenne Tour. Combining each of the pillars that make up PPMD’s mission, the End Duchenne Tour brings updates on research, advocacy, and care to cities across the country, featuring a roster of leading experts in the Duchenne space.
You have told us what topics are the most important to you and we have listened, using your feedback to inform our robust agenda at each tour stop. This is also an opportunity to connect with local families and, when possible, explore your area Certified Duchenne Care Center.
As always, each meeting is free with breakfast and lunch provided. Kids are also welcome to attend and participate in PPMD’s Kids Track.
Upcoming 2018 Schedule*:
- Birmingham, AL – June 2, 2018
- Billings, MT – Remote
- Dallas, TX – October 13, 2018
- San Francisco Bay Area, CA – November 3, 2018
- Cincinnati, OH – November 17, 2018
PPMD will also be holding our Annual Conference in Scottsdale, AZ (June 28 – July 1).
*Registration typically opens 1-2 months prior to each event. Visit EndDuchenne.org/Tour for more details and make sure you are signed up to receive emails from PPMD to be notified when registration opens.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy


