
PPMD was excited to head to the Midwest for the second stop on the 2018 End Duchenne Tour. The event took place at Western Michigan University in Grand Rapids, Michigan, in partnership with Team Joseph, Little Hercules Foundations, and Noah’s Feat. There were close to 100 people in attendance, including families, PPMD staff, members of industry, and healthcare providers.
There is something so wonderful about folks from the Midwest…they are compassionate, friendly, “roll up your sleeves”, kind of people. The PPMD team felt so welcomed by the families.
PPMD & Community Partners
The day began with introductions around the room so we all had a chance to know a bit about everyone attending. I provided an overview of PPMD and our mission, describing our work within four pillars – Research, Care, Advocacy, and Education – and how the day’s agenda would touch on each of these pillars.
I then introduced our foundation partners for the Grand Rapids Tour, who provided information about the inspiring work they do:
- Team Joseph – Marissa Penrod started Team Joseph in honor of her son. She does amazing work both local and nationally funding research and supporting families.
- Marissa also discussed Team Joseph’s partnership with Little Hercules; both Marissa and Kelly Maynard (founder of Little Hercules) are working together to help families with navigating insurance (Little Hercules) and issues with daily living (Team Joseph).
- Noah’s Feat – Karen Stahler, a grandmother in Michigan, started Noah’s Feat. She is raising money through different avenues (swimming, T-shirts) to support research.
The Therapeutic Pipeline
PPMD’s Founding President and CEO, Pat Furlong, provided an overview of current research strategies in Duchenne and a look at the therapeutic pipeline.
Genetics, DuchenneConnect & Your Family
Ann Martin, PPMD’s Certified Genetic Counselors who leads PPMD’s DuchenneConnect Registry, discussed the importance of genetic testing and the array of programs PPMD offers through the registry.
- Take home:
- Decode Duchenne is a free genetic testing program.
- PPMD’s patient registry needs families to update their profile at least twice a year! If you are not part of the registry, join today.
- Download Presentation Slides
Race to End Duchenne
Matt Dague – uncle of a young man with a Duchenne and a longtime participant in our endurance program, spoke about Race to End Duchenne and the upcoming Metro Health Grand Rapids Marathon on October 20 & 21. Matt encouraged attendees and their family and friends to join him at this race – which features a Full Marathon, Half Marathon, Full Marathon Relay, 10K and 5K – and raise funds and awareness to help end Duchenne.
PPMD’s Connect Michigan
We also heard from our local volunteer PPMD Connect Michigan Coordinator – Janelle Lundy – who is looking for other moms and dads to join and help lead the Connect MI group for support and social connection. If you are not linked into the MI group please contact PPMD’s Nicole Herring who can connect you with Janelle.
Care Highlights
Pulmonary
Jonathan Finder, MD spoke about the standards of care for pulmonary (lung) function.
- Take home: Dr. Finder emphasized the importance of breathing devices – cough assist, CPAP, BiPAP, and noninvasive ventilation. He said that a trach should not be necessary in most cases.
- Download Presentation Slides
Cardiac
Katherine Gambetta, MD (Lurie Children’s) discussed cardiology standards of care. She also discussed the benefits of steroids on the heart. Finally, she went over emerging devices– implantable cardioverter defibrillators (ICD) – if ejection fracture is <35%.
- Take home: Talk to your cardiologist about medications like Beta Blockers, ACES/ARBs and adding eplerenone to the ACE/ARB regimen.
- Download Presentation Slides
Physical Therapy
Laurey Brown, PT (Lurie Children‘s) discussed the stages of Duchenne based on ambulation. She went over the fundamentals of stretching with the goal of improving function, well-being, and prolong ambulation. She talked about letting children pace themselves. She stressed that fatigue is real; encouraged the incorporation of balance and coordination skills; explained how aquatic exercises are wonderful for Duchenne; and explained that in case of fractures, families should turn to the new care guidelines.
- Take home: A number of resources exist on the PPMD website and are available for both families and PTs.
- Download Presentation Slides
Industry Updates
Several companies presented on their products, providing trial updates:
- Catabasis (Joanne Donovan, MD) – Discussed Edasalonexent and the MOVE DMD trial which is in an ongoing Phase 2 trial, about to enter Phase 3. (Download Presentation Slides)
- Pfizer (Rodger Kobes, MD, PhD) – Discussed their myostatin inhibitor, Domagrozumab, which is in Phase 2, soon to be Phase 3; They also discussed Pfizer/Bamboo’s mini-dystrophin gene therapy trial. The trial is looking for males ages 5-12, now recruiting at four sites in the US (only three patients to start, then six, and eventually twelve total).
- PTC (Marie Brace) – Discussed their work with both ataluren (currently recruiting for Phase 3 and for biopsy trial) and Emflaza, the first and only approved steroid for Duchenne patients. They also went over their Sibling Program and Strive Grants.
- Roche (Kim Johnson, PharmD) – Talked about Roche (the US affiliate is Genentech), their RG6206 anti-myostatin finishing Phase 2, starting Phase 3. They said that Phase 2 had no significant safety concerns and Phase 3 now recruiting. (Download Presentation Slides)
- Santhera (Teresa Chu, PhD) – Discussed idebenone and their ongoing study in Duchenne called SIDEROS, which is currently recruiting 266 males with Duchenne on steroids. They informed the group about their new website TakeaBreathDMD.com which focuses on respiratory health. (Download Presentation Slides)
- Sarepta (Brett Billmeyer) – Discussed their Exon 53 and 45 trial called ESSENCE. They are actively recruiting for that trial and beginning a trial for their next generation exon skipping drug (PPMO) for Exon 51. They also discussed their new Route 79 Duchenne Scholarship program – 10 scholarships of up to $10,000 for the fall 2018 semester. The application deadline is May 31.
- Summit (Michelle Avery, PhD) – Discussed their utrophin drug in trial– ezutromid – an oral utrophin modulator. Ezutromid is currently in Phase 2. The program is called PhaseOut DMD and has 40 patients enrolled. After 24 weeks, they have good results. They are waiting on the 48 week results and are starting to plan next trial. (Download Presentation Slides)
About the End Duchenne Tour
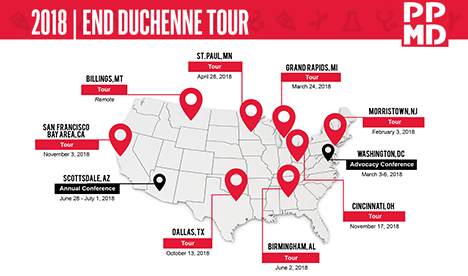
In an effort to reach every single family facing a Duchenne diagnosis in the U.S., PPMD has launched a multi-year community experience called the End Duchenne Tour. Combining each of the pillars that make up PPMD’s mission, the End Duchenne Tour brings updates on research, advocacy, and care to cities across the country, featuring a roster of leading experts in the Duchenne space.
You have told us what topics are the most important to you and we have listened, using your feedback to inform our robust agenda at each tour stop. This is also an opportunity to connect with local families and, when possible, explore your area Certified Duchenne Care Center.
As always, each meeting is free with breakfast and lunch provided. Kids are also welcome to attend and participate in PPMD’s Kids Track.
Upcoming 2018 Schedule*:
- St. Paul, MN – April 28, 2018
- Birmingham, AL – June 2, 2018
- Billings, MT – Remote
- Dallas, TX – October 13, 2018
- San Francisco Bay Area, CA – November 3, 2018
- Cincinnati, OH – November 17, 2018
PPMD will also be holding our Annual Conference in Scottsdale, AZ (June 28 – July 1).
*Registration typically opens 1-2 months prior to each event. Visit EndDuchenne.org/Tour for more details and make sure you are signed up to receive emails from PPMD to be notified when registration opens.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy






