As vaccines become available to help protect against COVID-19, PPMD is here to help you find answers to questions you might have about them.
Here, with the help of Drs. Tim Cripe and Tim Franson, we’ll provide an overview of the current authorized COVID-19 vaccines in the United States and discuss how mRNA vaccines work.
Please visit PPMD’s COVID-19 Information Center for additional resources and submit questions to ellen@parentprojectmd.org, so we can continue to adapt our resources to your concerns.
How conventional vaccines work
Typically, vaccines inject antigens, which is what your body makes to fight off a virus. This antigen is specific to the virus that the vaccine hopes to prevent you from getting.
How the new mRNA-based COVID-19 vaccines are different
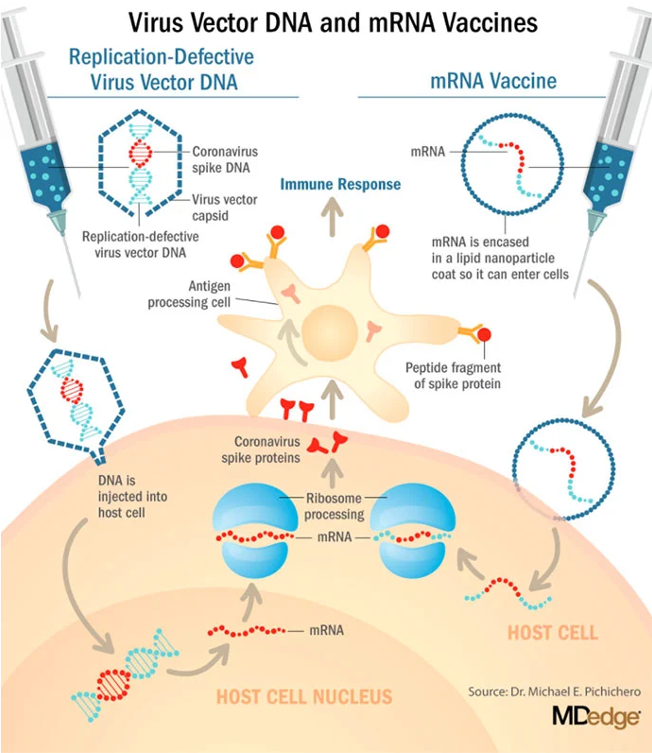
The new mRNA-based COVID-19 vaccines currently authorized in the US are a little different from conventional vaccines. The vaccines developed by Pfizer and Moderna both include genetic material consisting of messenger RNA (mRNA) particles that encode for the spike protein from the COVID-19 vaccine encased in a lipid nanoparticle (tiny fat bubble) that allows the mRNA to remain intact and keep the fragile mRNA from being degraded.
Injecting the mRNA instructs your body’s cells to make a piece of the spike protein from the virus. Each time the body’s cells replicate this tiny piece of the spike protein, it is alerted that there is now a foreign protein in the body and is prompted to create antigens to the protein.
By developing its own antibodies to the COVID-19 spike protein, the body mounts an immune response to that protein, protecting the body against the invader. This process is illustrated in the image below.
Vaccines Currently Available for COVID-19
| Pfizer | Moderna | Janssen Biotech/Johnson and Johnson | |
|---|---|---|---|
| Vaccine Platform | mRNA encased in lipid nanoparticles (fat bubbles) to keep it from degrading | mRNA encased in lipid nanoparticles (fat bubbles) to keep it from degrading | Recombinant, replication-incompetent human adenovirus type 26 vector |
| Age of Inclusion | 5 years + | 18 years + | 18 years + |
| Accessibility | Storage between -94 degrees Fahrenheit (institutional research freezers), stable x5 days after thaw | -4 degrees Fahrenheit (regular freezer), stable x30 days after thaw and at room temperature for 12 hours | 36 degrees Fahrenheit to 46 degrees Fahrenheit (regular refrigerator); or 47 degrees Fahrenheit to 77 degrees Fahrenheit (room temperature) for up to 12 hours |
| Dosing Schedule | 2 injections given 21 days apart; 30µg dose given for 12+ and 10µg given for 5-11yrs | 2 injections given 28 days apart | Single dose |
| Efficacy | 95% effective | 95% effective | 85% effective in preventing severe/critical COVID-19 occurring at least 28 days after vaccination, 100% effective in preventing death |
| Possible Side Effects | Pain at injection site, fatigue, mild fever, headache, muscle pain, joint pain, fever; younger adults have reported more side effects than older adults. *In 5-11 year olds, more redness and swelling at injection site, and less frequent/milder fever. | Pain at injection site, fatigue, mild fever, headache, muscle pain, joint pain, fever; younger adults have reported more side effects than older adults | Pain at the injection site, headache, fatigue, muscle aches and nausea |
| Additional Doses | Yes, if moderately or severely immunocompromised. Administer at least 28 days after the second dose. Ages 5+. | Yes, if moderately or severely immunocompromised. Administer at least 28 days after the second dose. Ages 18+ | Unknown |
| Booster Doses | Yes, for people 12 years and older, five months after completion of primary series. **Booster shots may be mixed and matched. You can receive the same type you originally received, or receive a different type. CDC recommendations now allow for a mix/match approach to booster administration. | Yes, for people 18 years or older, six months after completion of second (or third) dose, at one-half the original dose. **Booster shots may be mixed and matched. You can receive the same type you originally received, or receive a different type. CDC recommendations now allow for a mix/match approach to booster administration. | Yes, for all people 18+, at least two months after first dose. **Booster shots may be mixed and matched. You can receive the same type you originally received, or receive a different type. CDC recommendations now allow for a mix/match approach to booster administration. |
| Approval | Full FDA approval for 18+; Emergency Use Authorization for 5-11 and 12-17 | Emergency Use Authorization | Emergency Use Authorization |
* Table reflects COVID-19 vaccines that have received emergency use authorization from the US Food and Drug Administration (FDA) as of January 15, 2021.
Of the two vaccines currently authorized, is one better to be given to people with Duchenne?
There is no evidence to suggest that one vaccine is safer or more effective than another. Both Pfizer and Moderna utilize the same type of vaccine platform, and neither should have any impact on RNA therapies or access to clinical trials.
Vaccines Not Yet Approved
- The AstraZeneca vaccine, which is NOT yet approved, uses a viral vector (adenovirus) that carries the genetic instructions from the virus’s DNA to the cells of the body to make a limited supply of the full COVID-19 spike protein. The body then sees the foreign spike protein and develops its own antibody to this foreign visitor. The Johnson and Johnson vaccine is similar to AstraZeneca but uses a different adenovirus, adenovirus 26. Both vaccines are being studied in both one and two injection dosing schedules. A similar vaccine from a Russian company (Sputnik V) is also earlier in its development.
- Novavax, Sanofi, and GlaxoSmithKline vaccines, also NOT yet approved. Novavax uses “virus-like” particles that are housed in a viral vector (baculovirus). These genetically engineered virus-like nanoparticles are injected into the body, are seen as foreign and mount an immune response. Sanofi and GlaxoSmithKline are similar, but include a protein subunit from the COVID-19 spike protein that is injected and mounts an immune response.
- Sinovac and Sinopharm vaccines, also NOT yet approved, are planning to inject whole, inactivated or “killed” virus into the body that will mount an immune response. This is similar to the inactivated vaccines that we give to children, as well as the influenza vaccine.
Staying Safe & Making Informed Decisions
There are, of course, many things we do not yet understand about either of the vaccines currently authorized (i.e., how long does immunity last, will vaccines need to be boosted, etc.). While we are answering these questions, it is important to keep doing all the things we have been doing to keep ourselves and each other safe – social distance, wear your mask, wash your hands, stay home if you are sick.
As more vaccines are authorized, PPMD will continue to share updated information. Please let us know if you have any questions. Being informed and talking with your healthcare provider is the best way to make any decision.
Watch: COVID-19 Vaccination & Duchenne – What You Need To Know
For more information on COVID-19 vaccines and their development, as well as their direct impact on Duchenne, please view this webinar featuring Drs. Tim Franson and Tim Cripe:
SURVEY: IMPACT OF THE COVID-19 PANDEMIC ON DUCHENNE
PPMD has launched our second COVID-19 Pandemic Impact Survey related to your experiences during the pandemic for accessing care, trials, and approved therapies. Our latest survey delves into new questions around telemedicine experiences, as well as updated questions on trials and approved therapies. Please help us by completing this survey, whether or not you participated in our earlier spring survey. The data you provide through these surveys is vital to informing and shaping the work of relevant stakeholder groups in the Duchenne and Becker community, including physicians, drug developers, and patient advocacy groups.
Complete Survey >


 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

