Why we need the BENEFIT Act?
In 2012, Congress passed groundbreaking legislation called the The Food and Drug Administration Safety and Innovation Act (FDASIA) mandating that the FDA take a more structured and transparent approach to incorporating the views and perspective of patients and families into their decision making.
In order for FDA to make public how they evaluate benefit and risk into their decision making, FDA created a benefit-risk decision making framework that they fill out and now include in the public facing documentation:
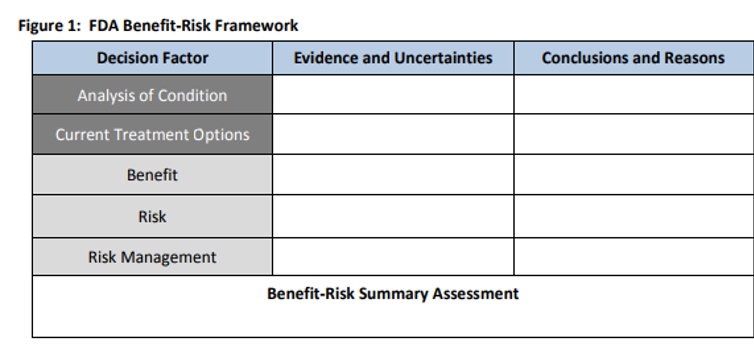
While progress has been made since 2012 on FDA incorporating the views of patients and caregivers into the drug approval process some significant gaps remain. One such gap is the lack of any requirement in law today that the FDA explain HOW they incorporated the views and perspectives of patients and caregivers into their final decisions on approved therapies. This means that the agency’s signature tool for evaluating benefit-risk (above) does not have to include data from the patient perspective that could be critical to informing the agency’s evaluation and, ultimately, decision on whether or not to approve a product.
To address this gap, Senators Roger Wicker (R-MS) and Amy Klobuchar (D-MN) and Representatives Doris Matsui (D-CA) and Brad Wentrup (R-OH) have introduced the Better Empowerment Now to Enhance Framework and Improve Treatments or the BENEFIT Act (S.373 and H.R. 4472).
The legislation would amend the Food, Drug and Cosmetic Act (FDCA) to ensure that patient experience, patient-focused drug development (PFDD), and related data – including information developed by a product sponsor or a third party such as a patient advocacy organization or academic institution – be considered as part of the benefit-risk assessment. This action would send an important signal to all stakeholders that patient experience and PFDD data will be fully incorporated into the agency’s review process and would encourage such entities to develop scientifically rigorous and meaningful tools and data.
History on the need for the BENEFIT Act
2012 – Patient Focused Drug Development
As mentioned above, because of the passage of FDASIA, the FDA now has to be more transparent about how they incorporate patient perspectives into their regulatory decision making. With the passage of FDASIA, FDA adopted a new program called the Patient Focused Drug Development (PFDD) initiative. Since the launch of PFDD, FDA has held meetings with patient groups (called listening sessions) and have been more intentional about including patient representatives into their ongoing work. FDA is also currently producing several guidance documents with clear instructions on how to best collect data directly from patients and caregivers about their diseases – also known as “patient experience data”.
2016 – Patient Focused Impact Assessment Act
After developing our own set of patient experience data for regulators and drug companies, PPMD was interested in understanding whether this data was reaching the very people who were reviewing potential New Drug Applications for Duchenne at FDA. In our view, there was a lack of transparency on how these tools were being used (or not used) by FDA reviewers and if the data was making it to the end of the drug development process. In 2016, PPMD, worked with our champions in Congress and proposed legislation called The Patient Focused Impact Assessment Act, this would essentially be a checklist of items FDA reviewers reported to have access to at the time of a product review. Because of our community’s advocacy efforts along with partners in the rare disease community, PFIA began a part of a larger FDA related bill called the 21st Century Cures Act which was passed into law in 2016.
Example of the PFIA checklist called “Statement of Patient Experience” 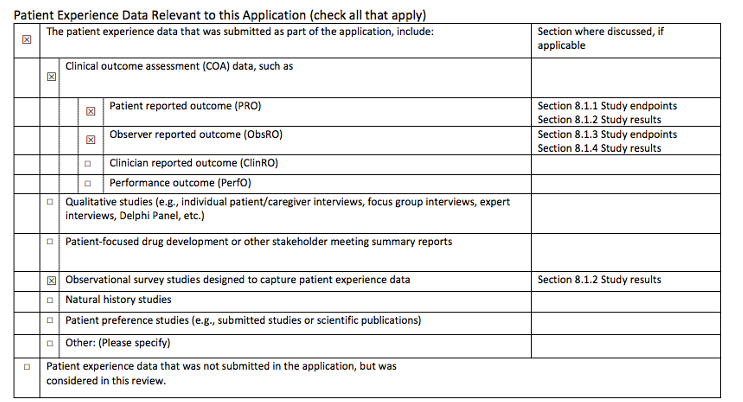
2022 – How the Better Empowerment Now to Enhance Framework and Improve Treatments (BENEFIT) Act would work
With the check list in place, today PPMD still sees a gap in the process. The gap – HOW is FDA actually INCORPORATING the patient voice into their decision making? As noted earlier, the FDA uses a signature tool when evaluating Benefit and Risk of emerging therapies called the Benefit Risk framework (see below). With each approved therapy they are required to fill out this matrix providing the public with transparency on how they evaluated benefit and risk in the context of a new therapy.
Our proposed change to this tool is modest, but will provide more clarity on how the PFDD or Patient Experience Data is used in their decision making.
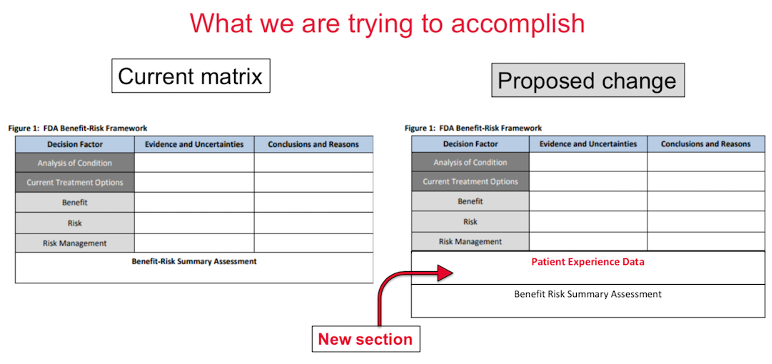
With this modest change, the patient community will learn more about how the experience of patients is weighed into the decision making of approved therapies.
The BENEFIT Act is supported by over 50 patient advocacy organizations. See a stakeholder letter in support of the bill here.