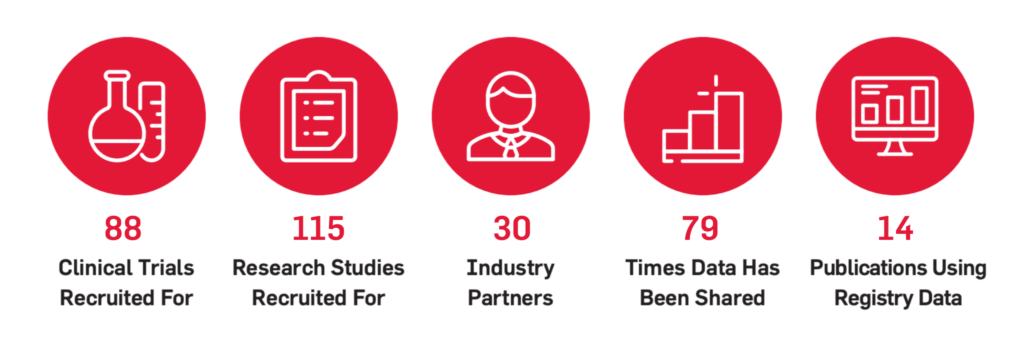
Everyone can help advance the research evolution for Duchenne and Becker. Bringing data sources together from multiple research endeavors is a key component of PPMD’s plan to advance the research evolution, and The Duchenne Registry has the ability to represent all families in this effort.
Started in 2007, The Duchenne Registry is PPMD’s patient-driven research program that allows the community to supply their own data directly to research. Patients and families share information about their diagnosis journey, test results, health conditions, and treatments to the Registry, which is stewarded by PPMD. De-identified Registry data is then used to support academic and industry researchers who use this information to better our understanding of Duchenne and Becker and the use of various therapies, as well as plan clinical trials.
The power of The Duchenne Registry is that everyone can play a role in its contributions to the community. PPMD hopes that you will:
JOIN The Duchenne Registry if you are eligible to participate. The Registry welcomes individuals with Duchenne, Becker, carriers, and anyone with a DMD gene variant, regardless of whether they have symptoms. The Registry is available in both English and Spanish.
SHARE about The Duchenne Registry to the audiences you connect with. We hope our industry partners share with the clinicians and researchers they interact with, that clinicians share with their patients, and that families share with each other.
STAY ENGAGED with The Duchenne Registry over time. Already 17 years old, the strength of the Registry is enhanced by persistence: continued awareness, continued participation, and continued support.
The Duchenne Registry is everyone’s way to contribute to the research evolution. Contact The Duchenne Registry team at coordinator@parentprojectmd.org for any questions, support needs for joining or updating an account, or for inquiries about data access or recruitment opportunities.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy