This week, PPMD’s Abby Bronson, SVP of Research Strategy and Eric Camino, PhD, Director of Research and Clinical Innovation are attending the annual World Muscle Society (WMS) meeting, this year in Copenhagen, Denmark. Below, Abby and Eric provide an overview of some of the updates they heard during the first day of the meeting.
Day 1: October 2, 2019
With over 900 attendees, World Muscle Society (WMS) 2019 opened October 2 in Copenhagen, Denmark at the famed Tivoli Gardens. With the Gardens decked out for Halloween, the mood is somewhat surreal and hopeful – very appropriate for all the promising science and research that is being presented at this meeting.
Plenary Sessions
The day started with plenary sessions focusing on very rare metabolic-based neuromuscular diseases, which seem unrelated to Duchenne – but there is much more to come. Highlights of the day were:
- A symposium sponsored by PTC Therapeutics that discussed the progression of Duchenne, clinically meaningful outcome measures and reviewed clinical and STRIDE Registry data from ataluren.
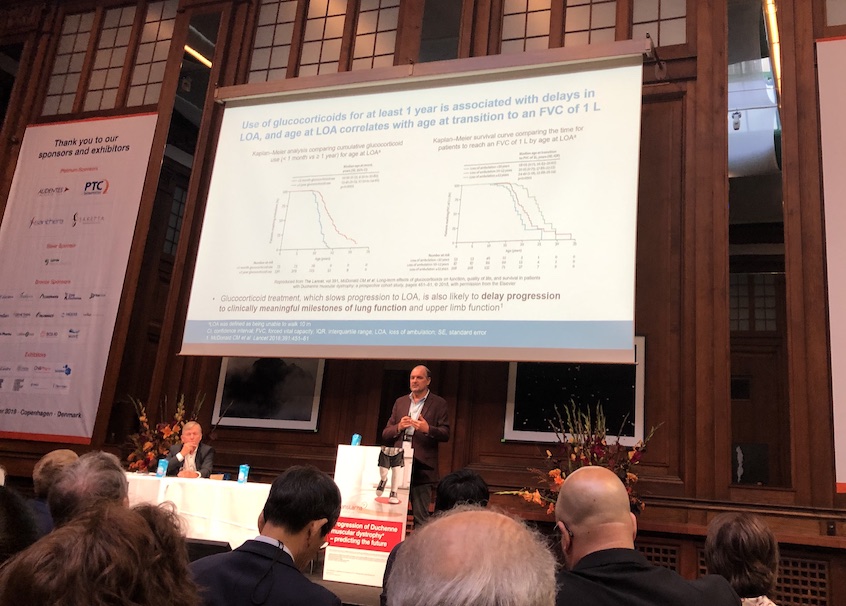
- A second symposium, sponsored by Santhera Pharmaceuticals, reviewed the natural history of respiratory function, outcomes as predictors of decline and standard of care for maintaining optimal respiratory function. Drs. Eugenio Mercuri, Craig McDonald, and Laurent Servais presented their data and answered questions on a range of topics spanning the benefits of continued steroid use after loss of ambulation and ways to track pulmonary function with at-home monitoring.
- And finally a session was devoted to molecular approaches to therapeutics, featuring CRISPR technologies that may be part of future treatment for those with premature stops in the DMD gene. Although CRISPR is still in the early days of development, it is exciting to see the creative ways in which researchers are applying these tools.
Poster Sessions
The posters sessions are absolutely packed with exciting Duchenne research. Abby and I look forward to really diving into the poster sessions more over the coming days. It’s wonderful and inspiring to see the innovative ways in which researchers are applying developing technologies to improve potential treatments. Highlights from some of the interesting projects we saw on the first day of the meeting include:
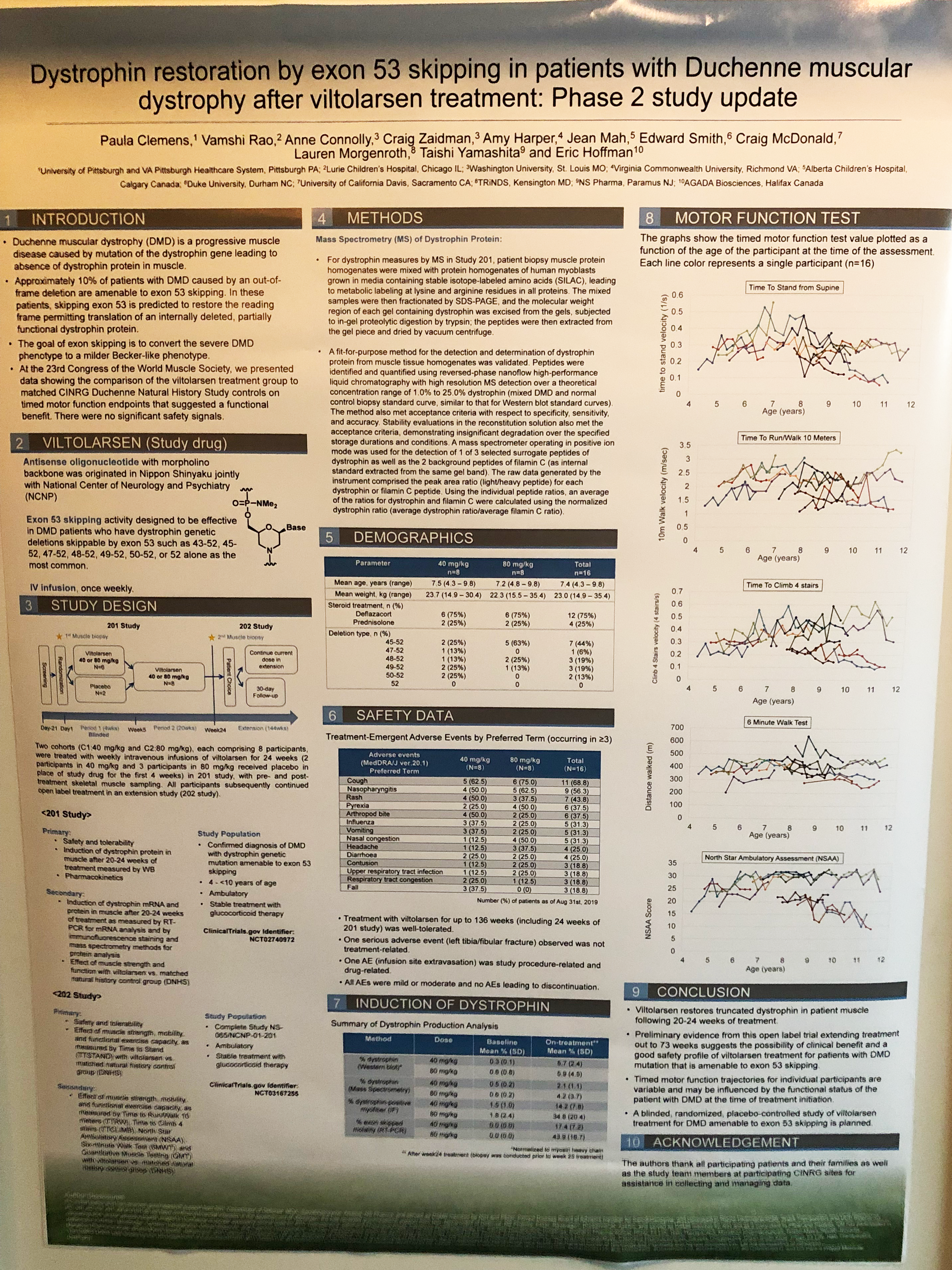
- NS Pharma, along with their exciting news that they had completed submission of their NDA to the FDA, had two posters showing results from their vitolirsen trial (press release: US and Japan).
- We also saw some interesting work from France using different drug combinations to impact gene therapy delivery.
Collaboration
The best part of meeting has been the opportunity to work with our colleagues at Duchenne UK. Earlier this week, we announced a joint grant our two organizations funded. Together, PPMD and DUK developed a booth to raise awareness about the power of the patient voice. Throughout all stages of drug development, the patient voice plays an important and impactful role. We want to ensure that sponsors, clinicians, and researchers recognize the voice of the patient and value added by listening to the community. We are highlighting all the great work this community has done to date to build infrastructure that has seen the pipeline of potential therapies for Duchenne continue to grow.
We hope to make new and lasting connections at WMS to brighten the future for all those with Duchenne. More updates soon!

What is World Muscle Society?
WMS was started 23 years ago by Professor Victor Dubowitz. Dr. Dubowitz led the Great Ormond Street, London clinic for many years. Dr. Dubowitz was one of the first to clinically adopt the use of steroids in Duchenne but, concerned about side effects, utilized a protocol of 10 days on and 10 days off. He also recommended the use of long leg braces (calipers) as a means to extend assisted ambulation well into the teenage years.
Dr. Dubowitz believes it is important for all individuals in the field to meet face-to-face to discuss opportunities, establish collaborations, and to connect at a forum showcasing findings, new investigators, and new knowledge. To that end, WMS and his journal, Neuromuscular Disorders, have grown to become incredibly important for the neuromuscular disease community. From relatively few investigators coming together to today, where over 750 people (bench scientists, young investigators, clinicians, companies, advocacy organizations, and investors) come together in different locations around the world, committed to driving therapies for neuromuscular diseases.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

