PPMD was thrilled to head to Las Vegas, NV for our first End Duchenne Tour stop of 2019 on February 2.
More than 45 people came together to learn about the latest information and resources for Duchenne families. PPMD was grateful to providers from the Las Vegas Clinic and University of Utah for spending their Saturday with us to connect with local families.
Here is a summary of the presentations:
Join the Fight
- Join the Race to End Duchenne Team at the 2019 Rock ‘n’ Roll Las Vegas Marathon on November 16 and 17: Run the Las Vegas Strip at night during this exciting evening race series! With a full marathon, half marathon, 10K and 5K, there is something for everyone. As a Race to End Duchenne team member, in exchange for your fundraising commitment, you’ll receive a number of benefits including a team celebration gathering, a personal fundraising page, training support and much more! For more information or to register, visit the Race to End Duchenne website.
- Stay in touch with the PPMD community by joining PPMD’s Connect, the official volunteer, parent-led outreach program of Parent Project Muscular Dystrophy. PPMD’s Connect groups serve as regional PPMD points of contact for families affected by Duchenne & Becker. The closest group to Las Vegas is currently PPMD’s Connect Southern California. To learn more or get involved, please visit the group on Facebook or contact Nicole Herring at nicole@parentprojectmd.org.
- The Decode Duchenne program, offering free genetic testing and counseling for people with Duchenne, is now available for carriers – learn more!

Research Updates
Research Overview
Eric Camino, PhD (PPMD)
- Eric discussed the importance of being a well informed family when deciding to participate in a clinical trial, with a reminder that clinical trials are experiments, not therapies.
- He presented the trials ongoing in the Duchenne pipeline: dystrophin restoration and replacement (i.e. exon skipping and gene therapy), muscle growth and regeneration (anti-myostatin), anti-inflammatory (steroid replacement drugs), and mitochondrial therapies.
- Download presentation slides
Industry Takeaways
- Catabasis – Joe Johnston
- Discussed Edasalonexant (CAT-1004), an oral therapy that inhibits NF-kB and the inflammatory response. Edasalonexant has shown improvement in biomarkers, muscle MRI, and functionality. Their Phase 3 POLARIS DMD trial is currently recruiting boys ages 4–7 at several sites in the US. Learn more at www.catabasis.com or www.clinicaltrials.gov.
- Download presentation slides
- Pfizer – Rodger Kobes, MD
- Discussed how PF-06939926 gene therapy uses a mini-dystrophin gene with a muscle-specific promoter targeted to cardiac and skeletal muscle. Now in Phase 1B and 5 boys already had the single infusion. Recruiting boys ages 5–12 years old, on steroids, at four US sites. Participants must test negative for antibody to viral capsid (probably ~30% of us have antibodies to this virus). Mainly testing safety in Phase 1 but also doing muscle biopsies to test dystrophin expression. Learn more at www.clinicaltrials.gov.
- PTC – Nick Mastrandrea, PhD
- Discussed Emflaza (approved in US) and Translarna (conditional approval in Europe). Also explained that PTC Cares is a program to help families receive access to Emflaza if they cannot get it covered through insurance. PTC is now recruiting for three different trials using Translarna. Learn more at www.ptcbio.com and www.clinicaltrials.gov.
- Download presentation slides
- Roche/Genentech – Mark Cabatingan, PhD
- Discussed RG6206 anti-myostatin therapy currently in a Phase 2 (SPITFIRE) study. RG6206 binds myostatin to block it from breaking down muscle and therefore encourage muscle growth. It is administered by a subcutaneous (under the skin) injection. Currently recruiting for SPITFIRE trial – looking for 159 ambulatory boys, any mutation, ages 6–11 years and on steroids. Many sites in US including Las Vegas. Learn more at www.clinicaltrials.gov.
- Santhera – Rebecca Persinger, PhD
- Discussed Idebenone, an investigational drug that targets the mitochondria and may improve pulmonary function. Completed DELOS Phase 3 trial for patients not on steroids, and currently still enrolling for SIDEROS trial for individuals on steroids. Participants must be age 10 or older and there are 64 centers worldwide. Visit www.siderosdmd.com and www.TakeABreathDMD.com to learn more.
- Download presentation slides
- Sarepta – Alison Craney, PhD
- Discussed Sarepta’s approved product (EXONDYS 51) for Duchenne and many other Duchenne therapies in their pipeline. Sarepta focuses on precision medicine, or products targeted to specific mutations. Alison explained the PMO and PPMO exon skipping platforms – PPMO is the second generation exon skipping which should unlock the full potential of the PMO exon skipping. Sarepta is also working in gene therapy and they have a Phase 2 trial (SRP-9001) that is recruiting 24 boys, ages 4–7 years old. It is placebo-controlled but the boys on placebo will get the infusion in Part 2. Learn more on www.sarepta.com or www.clinicaltrials.gov.

Care Highlights
Navigating the Challenges of School
Denise Gruender (ABC Educational Services)
- Denise offers solutions for parents to help children with academics (learning styles, homework, testing), independence, behavior, discipline, and many other issues.
- To reach Denise at ABC Educational Services, you may email her office manager at g@abcedservices.com or by phone: 704-443-2990.
Physical Therapy
Leslie Vogel, PT (Seattle Children’s Hospital)
- Leslie shared that exercise is safe, but moderation is important. Swimming is a great option.
- She discussed the importance of stretching and positioning as care management tools. She also covered the function of equipment that may be used at various stages of life from braces to standing chairs.
- Download presentation slides
Las Vegas Clinic
Jonathan McKinnon, MD and Kaitlyn Fell
- They have a bimonthly MDA Clinic and would welcome any patients from the Las Vegas area. They are experienced prescribing EXONDYS 51 and Emflaza, and they have three active clinical trials: Sarepta (NCT02500381), Roche (NCT03039686), and Mallinckrodt (NCT03400852).
- Download presentation slides
Neuromuscular Standards of Care
Russell Butterfield, MD, PhD (Univ of Utah)
- Butterfield discussed the updated Care Guidelines that were published in January 2018 in 3 journal articles. A Family Guide is available now as well. Dr. Butterfield reviewed all care areas and also discussed carriers – carriers need to get baseline echo/MRI with follow-up every 3–5 yrs because carriers can have significant cardiac issues.
- Don’t forget to use PPMD’s app for emergency care and the emergency care card. We also have a new tag for wheelchairs.
- In addition, Dr. Butterfield emphasized importance of Transitional Care for older teens and adults. They should not be followed by a pediatrician.
- Download presentation slides
Nutrition in Duchenne
Grace Gough, RD (Univ of Utah)
- Grace gave a fantastic talk on weight goals, calculating calories, nutrition recommendations, and swallowing complications. Some of her key take-home messages were:
- Weight management is easier than weight loss.
- Visit choosemyplate.gov. This site has great tips that are simpler than counting calories. For example, half of your plate should be fruits and veggies.
- If you must count calories, don’t do it in front of your child. And use the Schofield equation (you can Google it).
- Adopt family goals – everyone in your family should eat the same.
- Don’t forget calcium and vitamin D (almost everyone is vitamin D deficient), but most other supplements are NOT necessary if your child eats a healthy and balanced diet.
- Drink enough water. This is a big issue for people with Duchenne especially once they are non-ambulatory.
- Download presentation slides

PPMD & Community
About PPMD
Pat Furlong, CEO of PPMD
- Pat provided an overview of PPMD’s history in advocacy, the development of care guidelines, and growth of the Certified Duchenne Care Centers.
- She shared the power of advocacy on driving Duchenne care forward, as evidenced by the FDA’s published guidance on Duchenne drug development.
- PPMD’s annual advocacy conference is on the horizon, March 3 –5, 2019.
- Download presentation slides
Genetics, Your Family, and The Duchenne Registry
Ann Martin, MS, CGC (PPMD)
- Ann reviewed basic genetics and discussed The Decode Duchenne program which offers free genetic testing and counseling for people with Duchenne and Becker, and is now available for carriers – learn more.
- Ann also reviewed the importance of participating in The Duchenne Registry and how you can be a citizen scientist by joining and sharing your or your son’s data. Don’t miss the Ten Year Registry Report that was just released in January.
- If you have any questions about genetics, testing or clinical trials, please contact one of PPMD’s genetic counselors at 888-520-8675 or coordinator@duchenneregistry.org.
- Download presentation slides
PPMD’s Connect
PPMD’s Connect is the official volunteer, parent-led outreach program of Parent Project Muscular Dystrophy. PPMD’s Connect groups serve as regional PPMD points of contact for families affected by Duchenne & Becker. These groups offer:
- Family Mentoring
- Social Gatherings for Parents & Families
- Grassroots Outreach to support awareness & advocacy campaigns
- Fundraising Opportunities to support PPMD’s mission
The closest group to Las Vegas is currently PPMD’s Connect Southern California. To learn more or get involved, please visit the group on Facebook or contact Nicole Herring if you have any questions.

Race to End Duchenne
Join the Race to End Duchenne Team at the 2019 Rock ‘n’ Roll Las Vegas Marathon on November 16 and 17, 2019. Run the Las Vegas Strip at night during this exciting evening race series! With a full marathon, half marathon, 10K and 5K, there is something for everyone. As a Race to End Duchenne team member, in exchange for your fundraising commitment, you’ll receive a number of benefits including a team celebration gathering, a personal fundraising page, training support and much more! For more information and to register, go to http://join.parentprojectmd.org/lasvegas2019 or contact Nicole Herring if you have any questions.

Thank You!
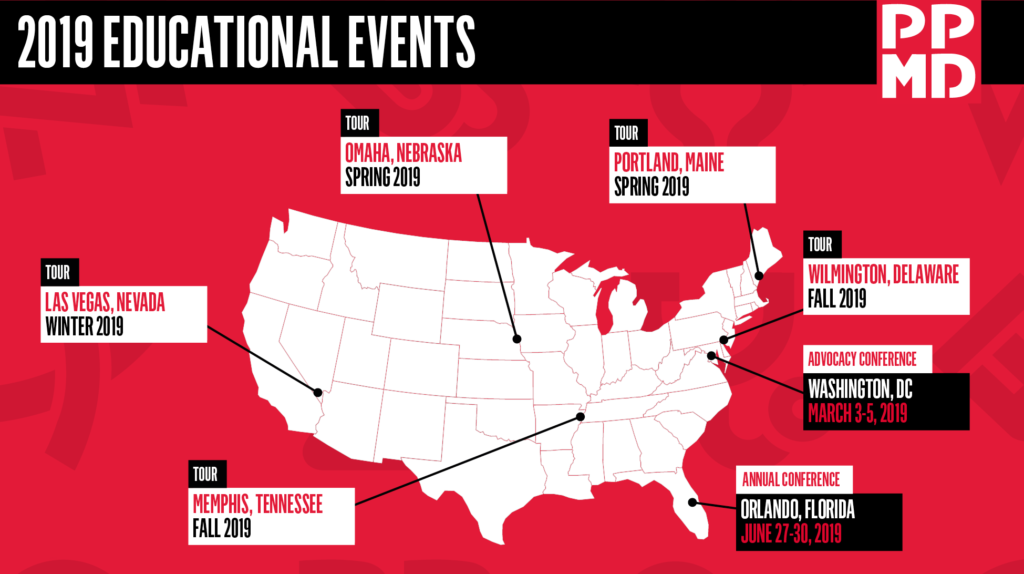
Thanks to the families, clinics and everyone else who helped make this day possible! Next End Duchenne Tour: March 30 in Omaha, Nebraska!
Next Up
In an effort to reach every single family facing a Duchenne diagnosis in the U.S., PPMD’s End Duchenne Tour brings updates on research, advocacy, and care to cities across the country, featuring a roster of leading experts in the Duchenne space.
You have told us what topics are the most important to you and we have listened, using your feedback to inform our robust agenda at each tour stop. This is also an opportunity to connect with local families and, when possible, explore your area Certified Duchenne Care Center.
As always, each meeting is free with breakfast and lunch provided. Kids are also welcome to attend and participate in PPMD’s Kids Track.
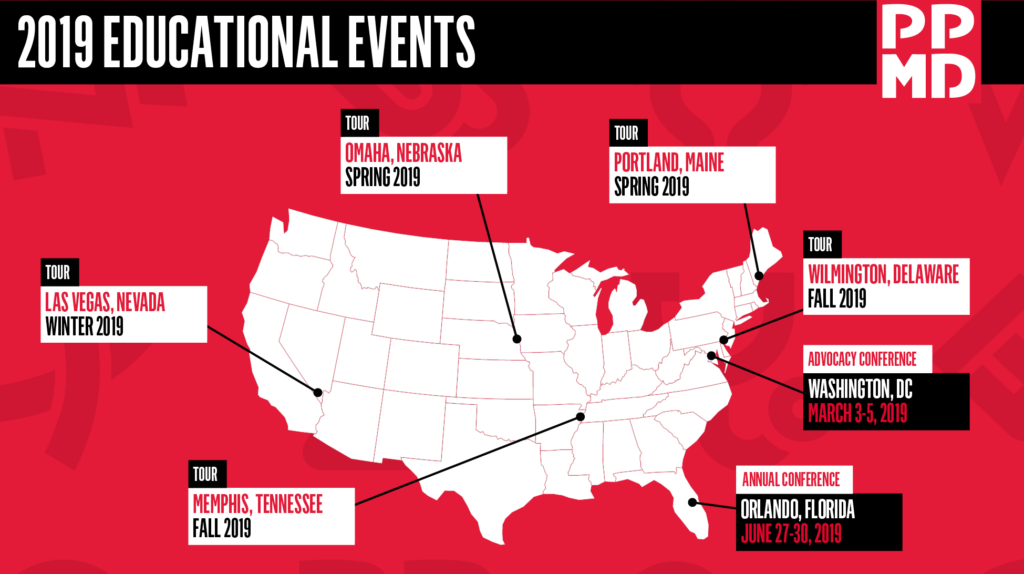
Upcoming 2019 Schedule*:
- Omaha, NE – Saturday, March 30, 2019
- Portland, ME – Saturday, April 27, 2019
- Wilmington, DE – Fall 2019
- Memphis, TN – Fall 2019
*Registration typically opens 1-2 months prior to each event. Visit EndDuchenne.org/Tour for more details and make sure you are signed up to receive emails from PPMD to be notified when registration opens.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy


