
PPMD is excited to announce the launch of our new Electronic Health Record (EHR) Study, which will extract data from several of our Certified Duchenne Care Centers across the country, with the first patients now consented to participate in the study.
Today marks an important next step in the development of PPMD’s Duchenne Outcomes Research Interchange (“the Interchange”) – a patient- and clinician-reported data warehouse which can combine data from The Duchenne Registry with EHR data, as well as data provided by industry partners with approved therapies.
In an effort to collect clinical data without adding additional burden on patients and families, once a patient agrees to participate in PPMD’s EHR study, important information will be automatically collected from doctor visits and sent to the Interchange. Collecting data in this way will allow doctors and researchers to better understand Duchenne and use that data to improve care and answer research questions in a way that does not require extra effort from you or your doctor.
By combining both patient-reported data from The Duchenne Registry, as well as clinician-reported data, the Interchange will provide a more complete picture of Duchenne and Becker muscular dystrophy, with the goal of helping researchers and clinicians to improve care and develop treatments faster.
Whose data will be collected from the EHR? How do I join the study?
If your Certified Duchenne Care Center (CDCC) is participating, you will be invited to join PPMD’s EHR Study at an upcoming clinic visit or over the phone. Arkansas Children’s Hospital is the first CDCC to participate, but several more CDCCs are in the process of joining PPMD’s EHR Study this year. In order to participate you must sign the EHR Study Informed Consent. No data will be collected from your EHR until you have signed the consent form.
What data will be pushed from the EHR?
If you consent to participate, your clinic will securely push data from the EHR to the Interchange. The data will include diagnosis, medications, laboratory tests, and procedures. Retrospective (past) data will be pushed, and all prospective (future) data will be pushed until the study ends or you request to be withdrawn from the study.
Where will the data be stored?
All of the EHR data will be stored in PPMD’s Duchenne Outcomes Research Interchange (“the Interchange”). This is also where all of the data from The Duchenne Registry is stored. PPMD launched the Interchange in 2018 with Prometheus Research (an IQVIA company), an industry leader in health data informatics. All data is stored securely and in accordance with strict industry standards and patient privacy laws. In addition, the EHR data is encrypted in transfer and at rest for enhanced security.
How is this different from The Duchenne Registry?
The Duchenne Registry is a self-report registry that has been collecting data directly from patients and families since 2007. This data is reported through The Duchenne Registry App via several medical surveys, and all the data is stored securely in the Interchange.
If you are actively participating in the Registry app, thank you! If you choose to also participate in PPMD’s EHR Study, your EHR data may be combined with your Registry data in the Interchange. Together, this information can help create a more complete picture of Duchenne.
If you have not yet joined The Duchenne Registry, now is a great time to join! Visit DuchenneRegistry.org and click Join to get started.
What is the ultimate goal for collecting this data?
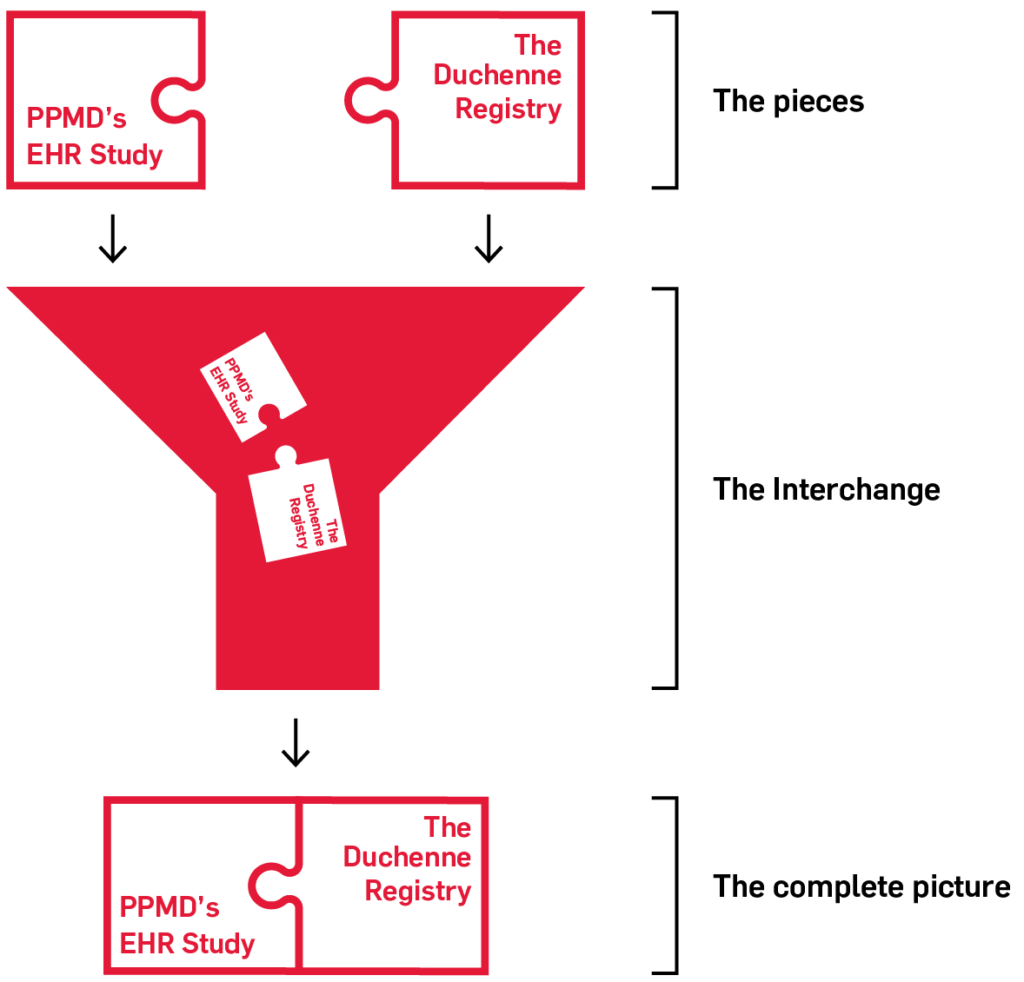 The goal is to combine your EHR data with the data you enter in The Duchenne Registry into one central place, the Interchange.
The goal is to combine your EHR data with the data you enter in The Duchenne Registry into one central place, the Interchange.
The Interchange can also house data from other sources, such as claims data from insurance companies and post-marketing surveillance data from industry partners with approved therapies.
The Interchange will provide a central warehouse for all types of Duchenne data and ‘real world evidence’ to be collected and analyzed in one place, which will benefit the entire Duchenne community. This will help clinical researchers and partners in therapy development address common research challenges and do their work better and faster, thereby accelerating scientific discovery and patient access to new therapies.
Collecting data from your EHR will also eventually reduce the burden on you, because we will not need surveys with questions about information that we can accurately get from the EHR. However, it will always be important to get other information directly from you about how you or your child are feeling and what they are able to do, so The Duchenne Registry remains a very important tool in Duchenne research.
TOGETHER, your EHR data and your Registry data are even more powerful in our fight to end Duchenne!
If you have any questions, please reach out to PPMD’s genetic counselors at coordinator@duchenneregistry.org or call 888-520-8675. We are available Monday – Friday, 8am – 5pm EST.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

