One thing we all know about Duchenne is that no matter when you or your child was diagnosed, some days we wake up and find ourselves feeling like there is still so much we need to learn and understand.
While 2020 has forced us to pretend to be comfortable with the unknown – taking one day at a time – I believe there are ways to dig deeper and decrease the uncertainty in Duchenne. Why is it that people with Duchenne progress so differently from one another? Is there any way for us to gauge how or when things will change? Or better yet, what might be able to stop or slow progression in a more meaningful way?
We can do this by exploring biomarkers in people living with Duchenne. Biomarkers are naturally occurring molecules, genes, or signals found in blood and urine that reflect changes in the body. For people with Duchenne, biomarkers can show what is happening in the disease, and how drugs and other therapies are working or not working.
Unfortunately, like so many things, it isn’t simple.
It’s not just measuring your blood pressure to determine if it’s too high or low. In order to identify relevant biomarkers for Duchenne we first have to determine if the markers are going to tell us something, provide insight and identify differences. Then we need to compare it to the other data sets we have generated and connect the dots about disease progression to determine what these markers can actually tell us.
Some History
In 2015, PPMD partnered with SomaLogics to address the critical need for useful biomarkers to help with the diagnosis and treatment of Duchenne. Using a machine called the SOMAscan, 1,125 proteins were measured simultaneously in the blood of Duchenne patients and healthy age-matched controls. The research group identified highly significant changes in the concentration of 44 different proteins, which were found to be either highly increased (24 proteins) or decreased (20 proteins). While several of these protein changes have been previously described, many of the other proteins discovered using this approach were unexpected and not previously associated with Duchenne.
Yetrib Hathout, PhD (Binghamton University) and colleagues have expanded on this initial study, identifying and confirming a variety of circulating protein biomarkers that reflect the complex nature of Duchenne.
We now have to take this initial work onto the next step in order to expand our set of tools for drug development. Our ultimate goal is to provide a set of diagnostic and predictive tests characterizing every person with Duchenne. These blood accessible biomarkers are useful to Duchenne clinicians, researchers, and patients as tools to assess disease progression and response to therapies
What’s Next
Our next step is to bridge these biomarkers to disease milestones and clinical outcomes in Duchenne in follow-up studies. That’s why we’re formally launching our Biomarker Initiative with the Duchenne Protein Mapping Project. The protein mapping project will bring together multiple data repositories that have years of samples that we can analyze. These samples may hold the key to determining if we can identify a protein map of Duchenne. If we can study protein differences in those who progress at different paces that may unlock our understanding of why progression differs for each individual and if certain individuals may respond better to potential therapies.
So what the heck is this protein map?
Well, first we will bring together years of patient clinical data, including imaging and functional data along with blood and urine collected during these studies. Then we’ll run those blood and urine samples through a proprietary scan that can pick up hundreds and hundreds of circulating proteins. Following identifying proteins, we will analyze and combine that information with the other information we know about the patients that contributed the data. From there, we will map out the correlation between protein levels detected and the relative timeline of progression to note evidence of any patterns.
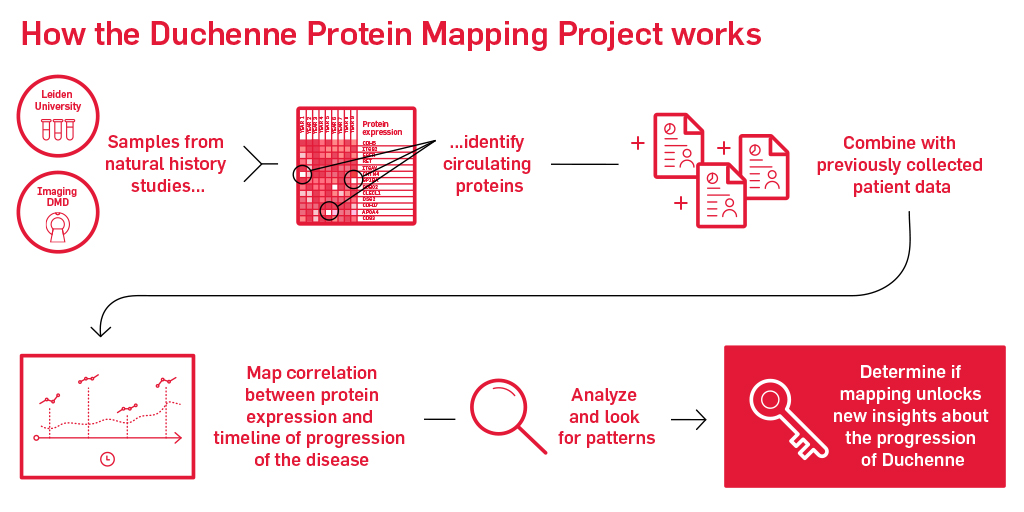
If we can map this, then we are one step closer to a disease progression protein biomarker for Duchenne. By refining the map of serum proteins for Duchenne, we will set the stage for future studies that will be better informed by and utilize serum biomarkers for the conduct and analysis of clinical trials.
This is a novel partnership that brings together Imaging DMD, Leiden University, and all those families who have contributed to various studies. We’ll rely on the proprietary technology of SomaLogic’s SomaScan and the expert AI enhanced analysis capabilities at Biosymetrics.
Support PPMD’s Duchenne Protein Mapping Project
This community, which never will cease to amaze me, through unparalleled citizen science and an unrelenting will, can help us understand better why Duchenne has such heterogeneity. We can take the contributions of a decade of study participation and data contribution to help define what therapeutic targets will be most effective for certain ‘types’ of Duchenne. Through, quite literally, your blood, sweat, and tears we may offer predictability to our entire community.
I hope you will donate to this project as we work to unlock data and unleash answers.
Donate >Special thanks to our community partners who generously supported the Duchenne Protein Mapping Project. Because of Team Joseph, Little Hercules Foundation, Ryan’s Quest, and Another Day for Gray, all donations will go twice as far.
 |  |  |  |
Upcoming Webinar
Join us on Wednesday, December 16 at 1 PM EST for an overview of PPMD’s Biomarkers Initiative, the amazing work from ImagingDMD, and the importance of leveraging data for new discoveries.
Register >


 by: Pat Furlong
by: Pat Furlong

