
This week, PPMD’s Abby Bronson, SVP of Research Strategy and Eric Camino, PhD, Director of Research and Clinical Innovation are attending the annual World Muscle Society (WMS) meeting, this year in Copenhagen, Denmark. Below, Abby and Eric provide an overview of some of the updates they heard during the second and third days of the meeting. You can read a recap from day one here.
Days 2 & 3
The second and third days of WMS featured a number of talks on the research ongoing in Duchenne.
Dystrophin and the Brain
The second day of WMS opened with a nice talk looking into the expression of dystrophin protein in the brain. The impact and role of dystrophin in the brain has not been as well characterized as skeletal muscle. By enhancing our knowledge of the relationship between dystrophin and the brain, will help to guide better treatment for those with Duchenne. While the talk was focused on dystrophin expression and its potential role, the following posters investigate the prevalence of neurodevelopmental symptoms in both Duchenne and Becker.

Gene Therapy
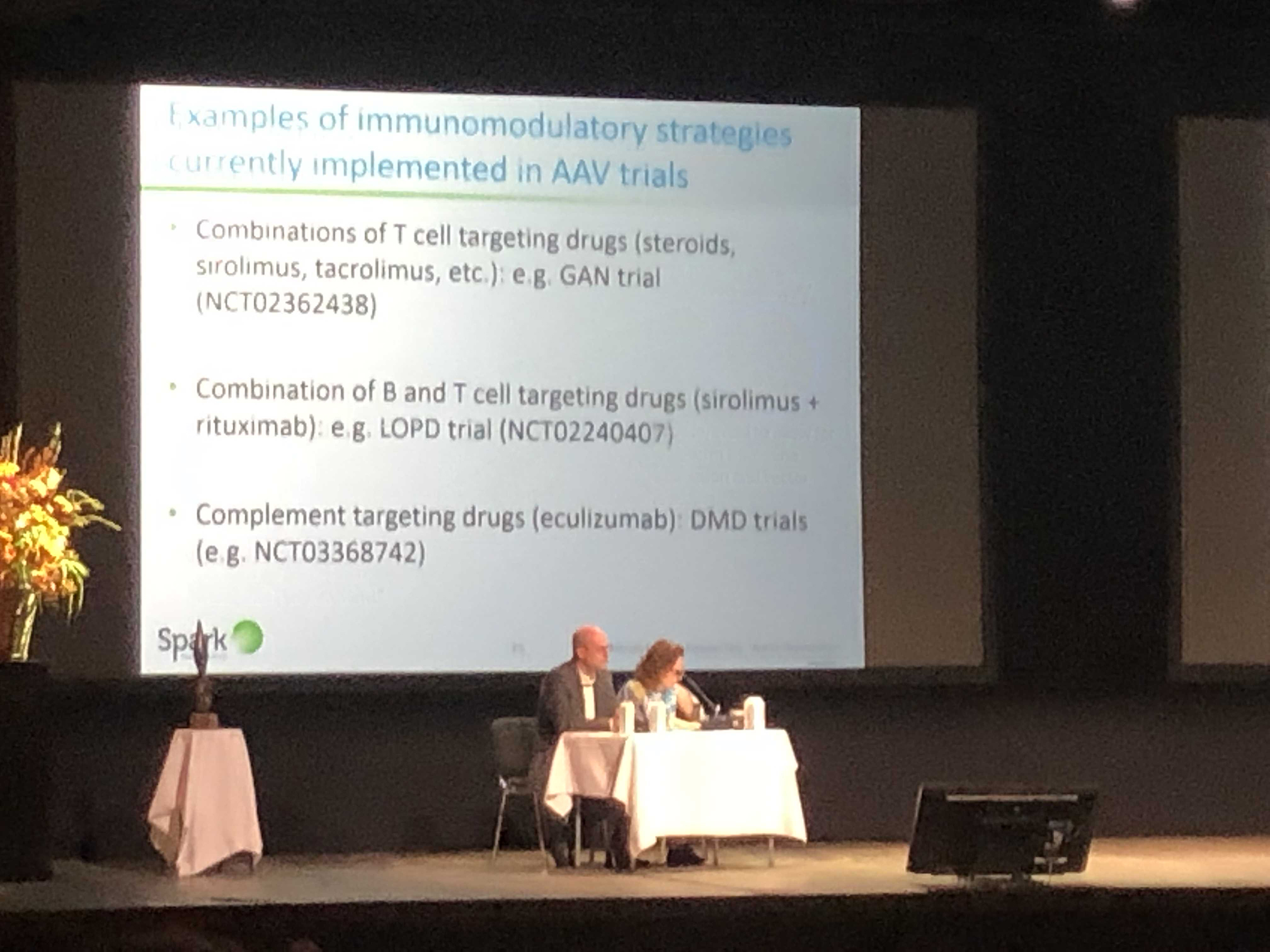
Day three featured an important talk on gene therapy, specifically AAV and the immune system. The immune system can make the use of viral vectors for gene therapy difficult. The challenges with pre-existing antibodies was discussed, as well as a number of efforts underway to circumvent these issue. These creative ideas are what is needed to help solve the issue to ensure all those with Duchenne can receive treatment.
In addition, Sarepta held a symposium that highlighted the success and safety of AAV in other diseases, which is promising for all the ongoing trials in Duchenne.
Poster Highlights
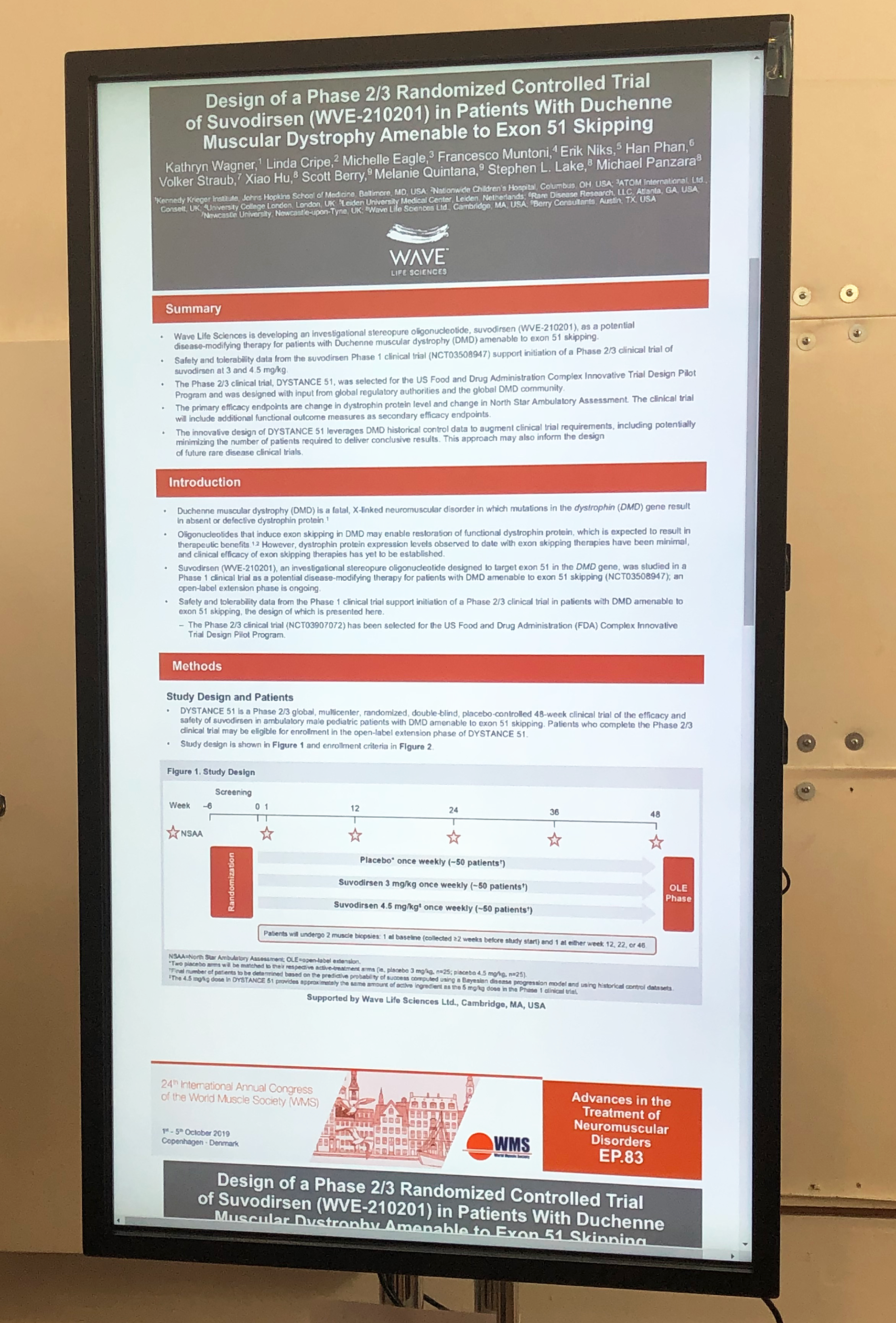
- Wave Life Sciences presented their phase 2/3 design which will have 3 arms randomized 1:1:1,, 3mg/kg, 4.5mg/kg and placebo. The trial will be 48 weeks in length and there will be two biopsies. This protocol is being designed as part of the FDA’s Complex Innovative Design (CID) pilot program and will be, in parallel, analyzing data from many natural history and placebo arms from external data sources in hopes that these will be robust enough to enrich the placebo arm, and adaptively reducing the number of placebo patients needed to conclusively analyze the trial results.
- Biophytis also presented an innovative statistical design using a composite measure, where measures of mobility, strength, and respiratory function will be combined in a composite measure. We will be watching both efforts closely.

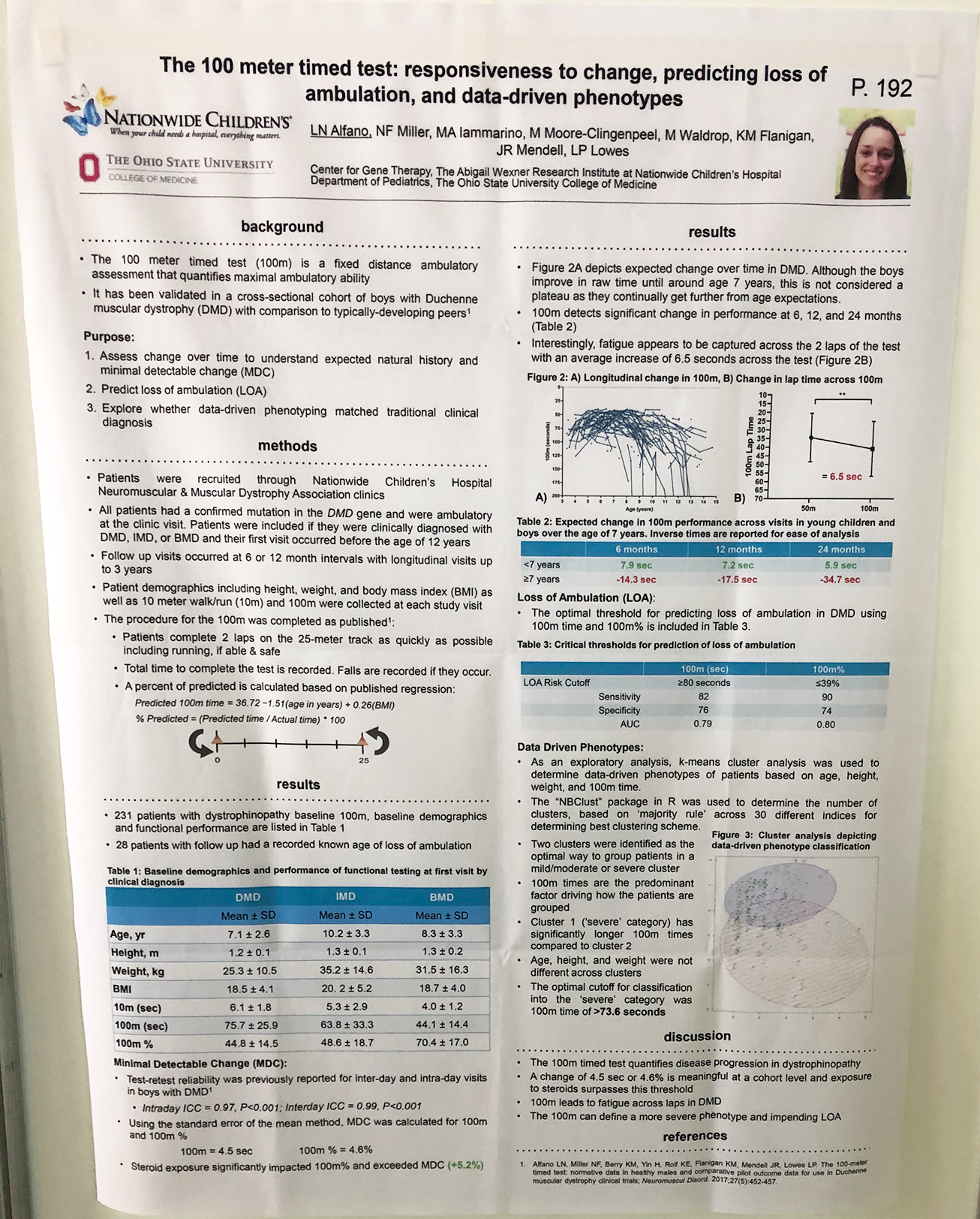
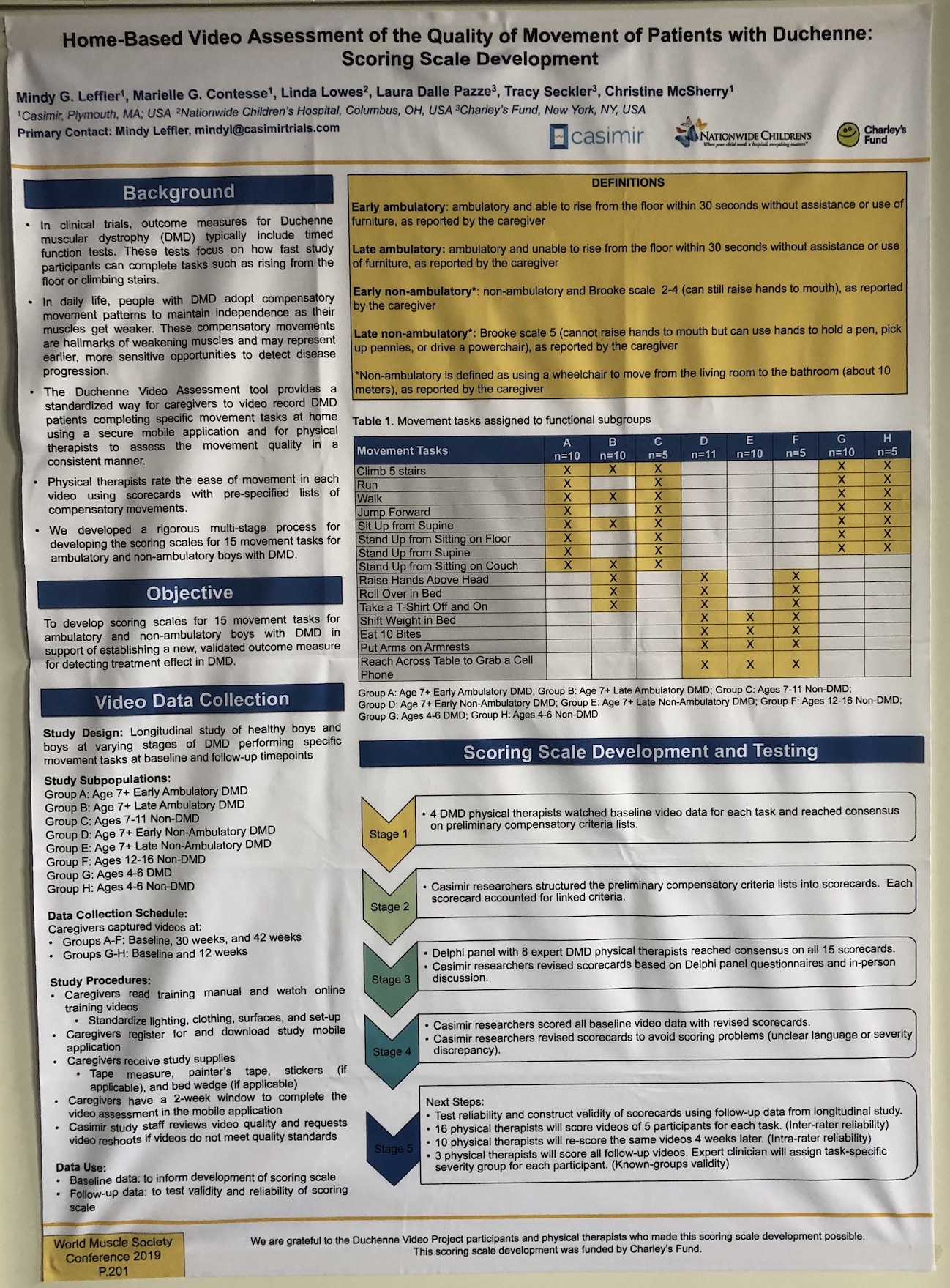
- There were many posters on novel outcome measures, from improving on existing measures to creating new ones, such as home video monitoring to ease trial burden on patients. The 100 m timed test – a measure that could be used instead of the 6 MWT as a measure of endurance – was shown to be responsive, predictive and reliable. We hope that this measure will provide a useful tool in clinical trials.
- D-RSC and C-PATH shared their work on disease progression models and enhancing clinical endpoints respectively, efforts that can allow for better trial design and outcome measures, which could help accelerate the trial process.
News from WMS
A number of our industry partners made announcements during the World Muscle Society meeting. Below is a list of those companies with a link to their announcements:
- Capricor Presents Additional Positive Data from Ongoing HOPE-2 Study of CAP-1002 in Duchenne Muscular Dystrophy at World Muscle Society: Data Demonstrates Improved PUL 2.0 Performance at 6 Months
- Catabasis Pharmaceuticals Presents Edasalonexent, A Potential Foundational Treatment For Duchenne Muscular Dystrophy
- NS Pharma: Morpholino Oligonucleotide NS-065/NCNP-01 (viltolarsen) Presentation of Phase II Study in the US
- NS Pharma: Morpholino Oligonucleotide NS-065/NCNP-01 (viltolarsen), Presentation on results of an additional analysis of Phase I/II study in Japan
- PTC Therapeutics Announces New Real-World Analysis Demonstrating Translarna™ (ataluren) Slows Disease Progression in Patients with Duchenne Muscular Dystrophy
- Santhera Announces Presentation by ReveraGen of Positive 18-Month Data with Vamorolone in Duchenne Muscular Dystrophy
What is World Muscle Society?
WMS was started 23 years ago by Professor Victor Dubowitz. Dr. Dubowitz led the Great Ormond Street, London clinic for many years. Dr. Dubowitz was one of the first to clinically adopt the use of steroids in Duchenne but, concerned about side effects, utilized a protocol of 10 days on and 10 days off. He also recommended the use of long leg braces (calipers) as a means to extend assisted ambulation well into the teenage years.
Dr. Dubowitz believes it is important for all individuals in the field to meet face-to-face to discuss opportunities, establish collaborations, and to connect at a forum showcasing findings, new investigators, and new knowledge. To that end, WMS and his journal, Neuromuscular Disorders, have grown to become incredibly important for the neuromuscular disease community. From relatively few investigators coming together to today, where over 750 people (bench scientists, young investigators, clinicians, companies, advocacy organizations, and investors) come together in different locations around the world, committed to driving therapies for neuromuscular diseases.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

