This week, PPMD’s Abby Bronson, SVP of Research Strategy and Eric Camino, PhD, Director of Research and Clinical Innovation are attending the annual World Muscle Society (WMS) meeting, this year in Copenhagen, Denmark. Below, Abby and Eric provide an overview of some of the updates they heard during the second and third days of the meeting. You can also read a recap from day one and days two and three.
Day 4
The final day of the World Muscle Society featured the most relevant talks to drug development for Duchenne. A number of other diseases also have gene therapies currently being deployed in their pipelines and it was encouraging to see similar methodologies trending towards success in those muscle diseases. Gene therapy programs utilizing AAV for limb-girdle muscular dystrophy, myotubular myopathy, and SMA were all presented. From the Duchenne pipeline vamorolone, edasalonexent, Cap-1002, and a new pre-clinical molecule all shared updates.
Vamorolone
Encouraging 18-month data from 23 patients in the open-label vamorolone trial was presented, showing efficacy similar to corticosteroids, with a good safety profile and reduced corticosteroid associated side-effects. A Phase 3 trial for vamorolone is currently recruiting.

Edasalonexent
Edasalonexent shared results from their MoveDMD trial, sharing safety data indicating growth curves are similar to normal with the stabilization of function out to 72 weeks, a Phase 3 trial (GalaxyDMD) for edasalonexent is currently ongoing, but has completed recruitment.
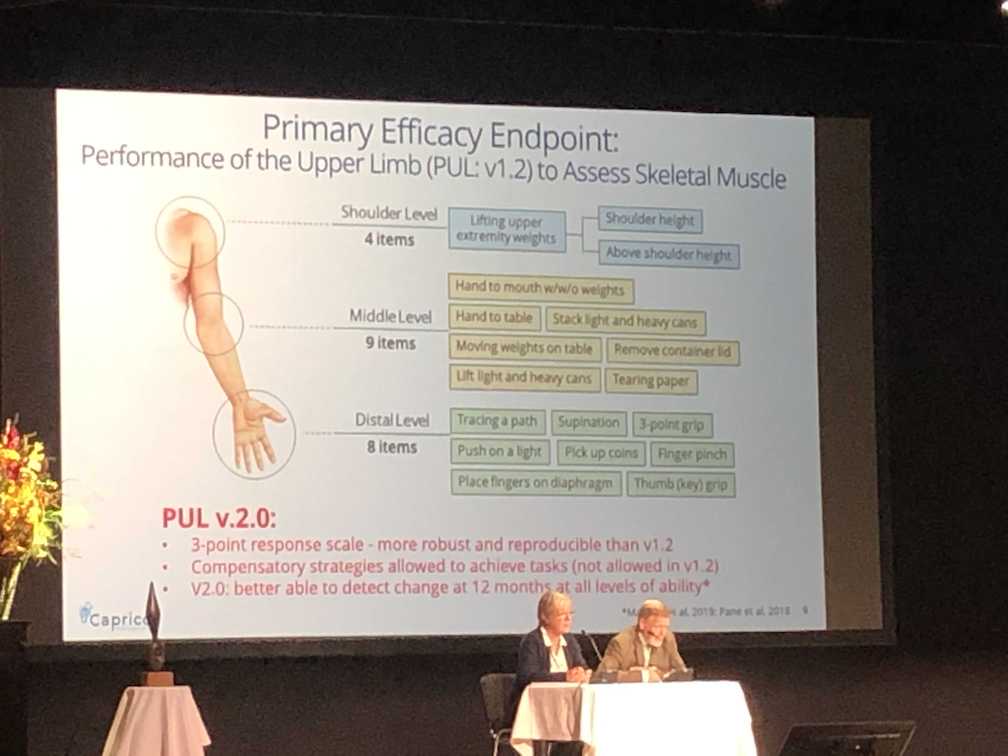
CAP-1002
Capricor Therapeutics presented interim data with a focus on evaluating upper limb functional improvements in non-ambulant patients with Duchenne using the PUL 2.0, an improved outcome measure for investigating upper limb function in Duchenne. The interim data suggests improvements in function, strength, pulmonary and cardiac endpoints; Capricor is planning to meet with the FDA to discuss if CAP-1002 could qualify for accelerated approval. We will be sure to keep working with Capricor to keep the community informed of their next steps.

Pre-Clinical Molecule
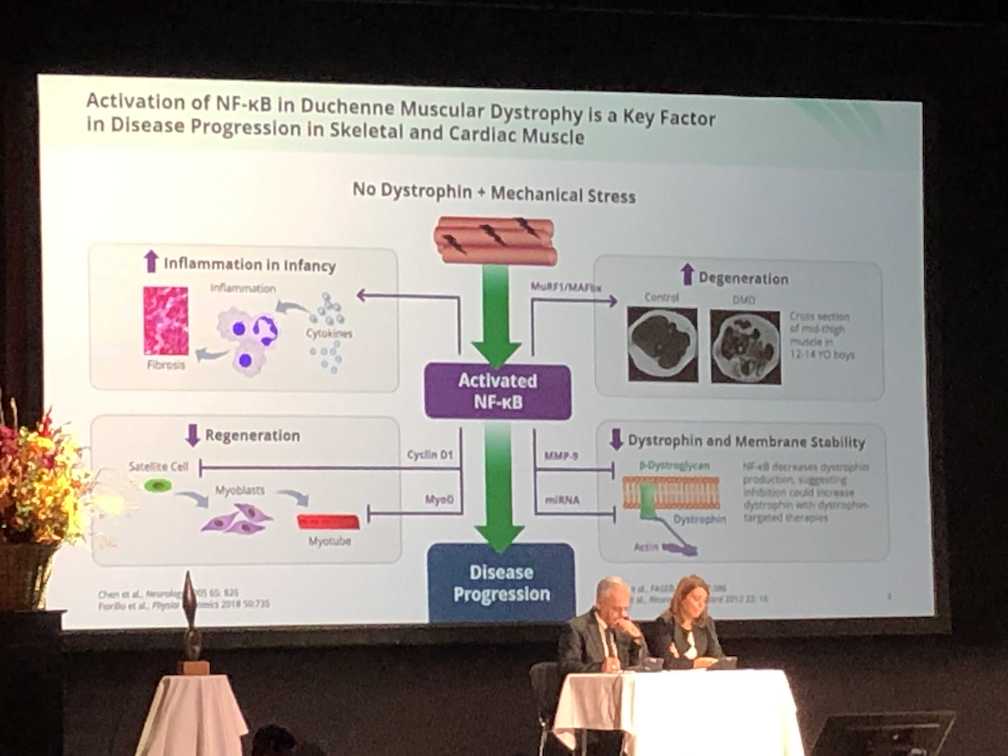
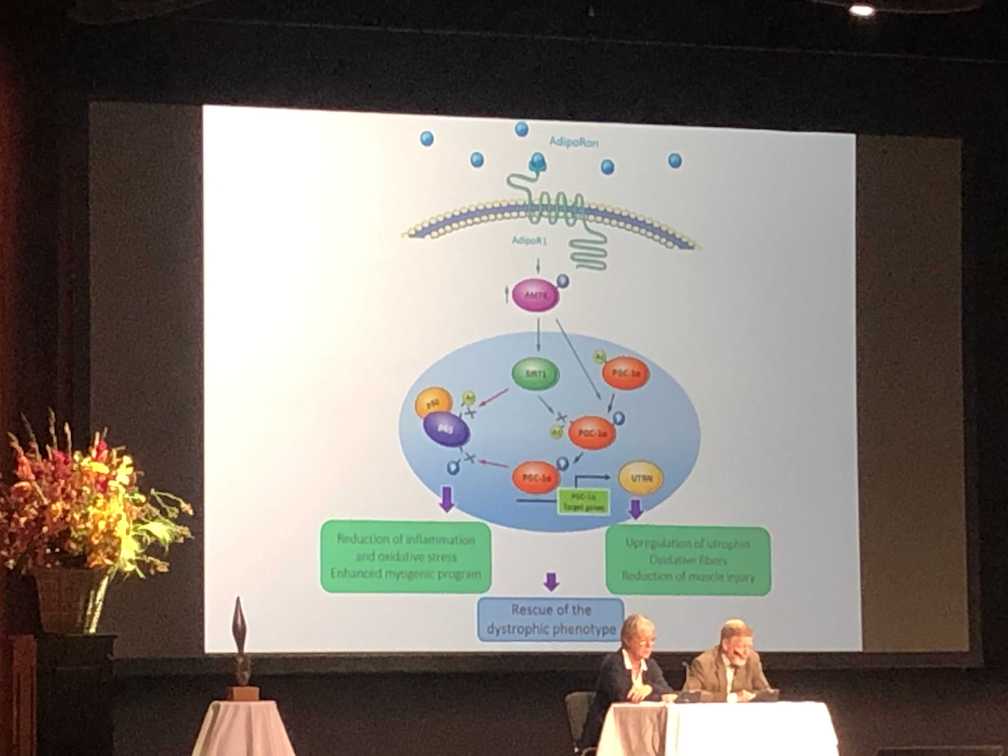
It was exciting to see a new compound being tested in pre-clinical models. AdipoRon, a synthetic version of ApN, activates AMPK, represses NF-Kb, and upregulates utrophin. This has the potential to reduce oxidative stress and inflammation, while promoting upregulation of utrophin, ultimately reducing muscle injury and promoting muscle cell growth. Testing will need to be translated to patients with Duchenne to assess if the potential therapeutic benefits are maintained in humans.
We are always hopeful when we see new therapies being developed for Duchenne. The work of this community has opened the door to a wealth of potential therapeutics to treat Duchenne, and we hope to continue building bridges with the community, our fellow foundations, industry, and regulators to bring treatments to all those living with Duchenne.
News from WMS
A number of our industry partners made announcements during the World Muscle Society meeting. Below is a list of those companies with a link to their announcements:
- Capricor Presents Additional Positive Data from Ongoing HOPE-2 Study of CAP-1002 in Duchenne Muscular Dystrophy at World Muscle Society: Data Demonstrates Improved PUL 2.0 Performance at 6 Months
- Catabasis Pharmaceuticals Presents Edasalonexent, A Potential Foundational Treatment For Duchenne Muscular Dystrophy
- NS Pharma: Morpholino Oligonucleotide NS-065/NCNP-01 (viltolarsen) Presentation of Phase II Study in the US
- NS Pharma: Morpholino Oligonucleotide NS-065/NCNP-01 (viltolarsen), Presentation on results of an additional analysis of Phase I/II study in Japan
- PTC Therapeutics Announces New Real-World Analysis Demonstrating Translarna™ (ataluren) Slows Disease Progression in Patients with Duchenne Muscular Dystrophy
- Santhera Announces Presentation by ReveraGen of Positive 18-Month Data with Vamorolone in Duchenne Muscular Dystrophy
What is World Muscle Society?
WMS was started 23 years ago by Professor Victor Dubowitz. Dr. Dubowitz led the Great Ormond Street, London clinic for many years. Dr. Dubowitz was one of the first to clinically adopt the use of steroids in Duchenne but, concerned about side effects, utilized a protocol of 10 days on and 10 days off. He also recommended the use of long leg braces (calipers) as a means to extend assisted ambulation well into the teenage years.
Dr. Dubowitz believes it is important for all individuals in the field to meet face-to-face to discuss opportunities, establish collaborations, and to connect at a forum showcasing findings, new investigators, and new knowledge. To that end, WMS and his journal, Neuromuscular Disorders, have grown to become incredibly important for the neuromuscular disease community. From relatively few investigators coming together to today, where over 750 people (bench scientists, young investigators, clinicians, companies, advocacy organizations, and investors) come together in different locations around the world, committed to driving therapies for neuromuscular diseases.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

